Figure 4.
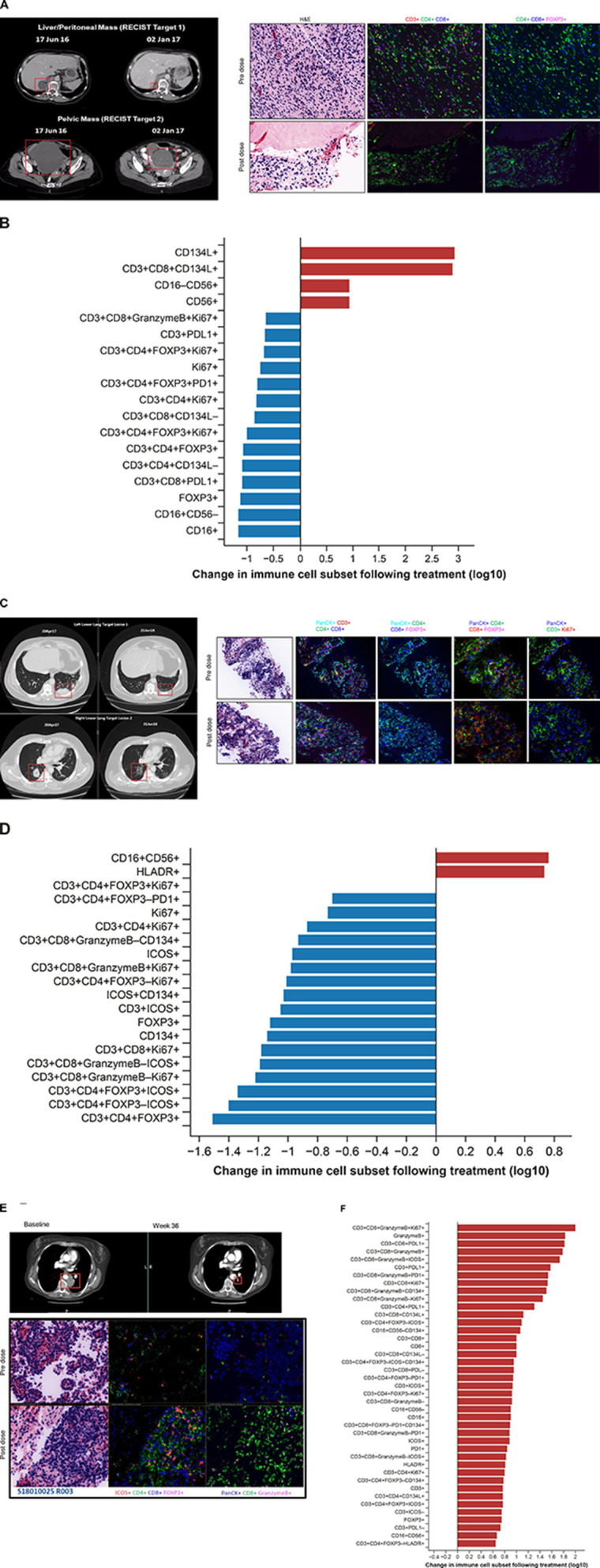
CT scans and multiplexed immunofluorescence analysis of immune cell infiltration in paired biopsies from responding patients. (A, B) GSK3174998 monotherapy (0.3 mg/kg dose)—dedifferentiated liposarcoma. (A) PD-(L)1-naive female patient in her mid-60s with dedifferentiated liposarcoma who remained on 0.3 mg/kg GSK3174998 monotherapy for 39 weeks. At the time her first scan was performed at week 12, her two target lesions (sum of diameters=167 mm) had decreased in size by 29% and further decreased by 38% at 24 weeks (baseline and week 24 scans are shown). The target lesion response was maintained; however, non-target lesion disease progression resulted in treatment discontinuation at week 39. Prior to receiving GSK3174998, the patient’s treatment was surgery, then metronomic doxorubicin (15 mg/kg weekly) ending 1 month before initiating treatment with GSK3174998. Multiplexed immunofluorescence analysis of baseline and week 6 paired tumor biopsy samples show decreased Treg and increased NK/NKT cell infiltration. (B) Changes in immune cell subsets in tumor tissue from baseline to week 6. Immune cells displaying >5-fold changes are highlighted. (C, D) GSK3174998 (0.1 mg/kg dose) + pembrolizumab (200 mg) – melanoma. (C) A male patient in his early 60s with melanoma treated with prior ipilimumab+nivolumab until disease progression after 14 months, ending treatment with nivolumab ≈2 months before initiating treatment with 0.1 mg/kg GSK3174998+pembrolizumab 200 mg. At week 4, the patient developed organizing pneumonia and treatment was held until week 9, when CT scans showed an 8% reduction in target lesions (sum of diameters=98); 12 weeks later, the target lesions had achieved CR, which was maintained for the duration the patient remained on study, completing the 2-year treatment period and remaining in follow-up until the study was closed (baseline and week 57 scans are shown). Multiplexed immunofluorescence analysis of baseline and week 6 paired tumor biopsy samples show decreased Treg and increased NK/NKT cell infiltration. (D) Changes in immune cell subsets in tumor tissue from baseline to week 6. Immune cells displaying >5-fold changes are highlighted. (E, F) GSK3174998 (0.3 mg/kg dose) + pembrolizumab (200 mg) – NSCLC. (E) PD-(L)1-naive female patient in her mid-50s with NSCLC (TPS=1%) with no activating mutations and who had received prior treatment with carboplatin, pemetrexed, and bevacizumab. She received 0.3 mg/kg GSK3174998+pembrolizumab 200 mg treatment for the maximum duration of 2 years. At her week-6 CT scan, the target lesion (54 mm) had decreased in size by 37% and continued to decrease to a maximum of 64%, maintaining a PR for the duration of the study (baseline and week 36 scans are shown). Multiplexed immunofluorescence analysis of baseline and week 6 paired tumor biopsy samples show increased infiltration of T helper cells, cytotoxic T cells, granzyme B–expressing CD8+ T cells, ICOS+ T cells, and proliferating cytotoxic T cells. (F) Changes in immune cell subsets in tumor tissue from baseline to week 6. Immune cells displaying >5-fold changes are highlighted. CD, cluster of differentiation; CR, complete response; FOXP3, forkhead box protein 3; H&E, hematoxylin and eosin; HLA-DR, human leukocyte antigen D related; ICOS, inducible T-cell costimulatory; NK, natural killer; NKT, natural killer T cell; NSCLC, non-small cell lung cancer; PanCK, pan cytokeratin; PD-1, programmed cell death 1 protein; PD-L1, programmed cell death 1 ligand; PR, partial response; Treg, regulatory T cell; TPS, tumor proportion score.
