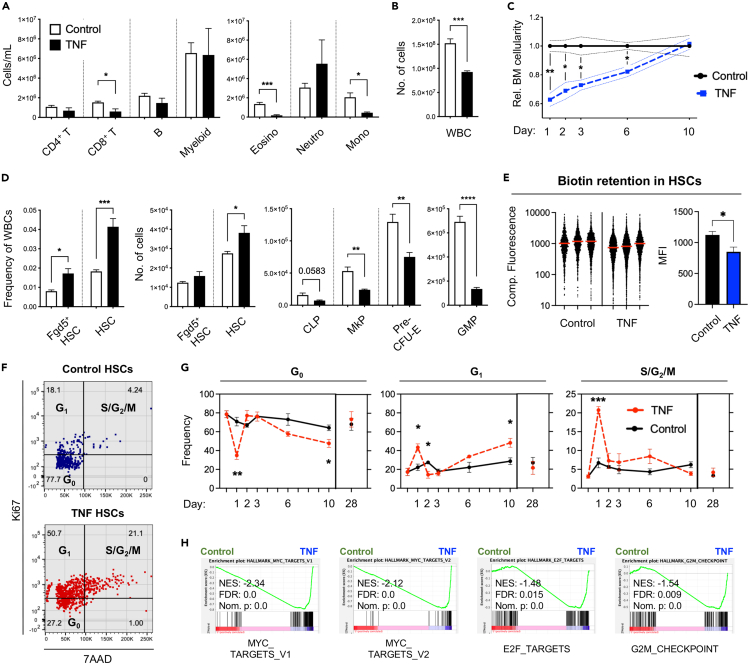
Figure 1.
Acute TNF exposure transiently decreases bone marrow cellularity and lineage-committed progenitors while increasing HSC proliferation and self-renewal
(A) PB cell concentrations of mature blood cell subsets.
(B) BM white blood cell (WBC) cellularity.
(C) BM WBC cellularity over time after TNF administration. Control n = 3, TNF n = 3 for all time points.
(D) HSC frequencies and cell numbers, and numbers of defined lineage-committed progenitor cells in the BM one day post TNF administration. Eosino = eosinophils, neutro = neutrophils, mono = monocytes. TNF-treated mice n = 5, control-treated mice n = 4.
(E) Proliferation history by biotin retention in HSCs two days post TNF administration. (Left) Compensated fluorescence distribution of biotin in HSCs. Red line represents mean. Control samples: 997, 1,187, and 1,709 events, TNF samples: 3,293, 4,228, and 2,262 events. 1,362 data points are outside the limits of the axis. (Right) Quantification of biotin data: mean fluorescence intensity (MFI) for each sample. Control n = 3, TNF n = 3.
(F) Representative cell cycle distribution plots for HSCs at one day post TNF exposure.
(G) HSC cell cycle distribution at various time points after TNF administration. Control n = 3, TNF n = 3 for all time points.
(H) Gene set enrichment analysis (GSEA) of proliferation-associated gene sets in HSCs 3 h post exposure to TNF. Error bars in A, B, D, and E represent +SEM. Dotted lines in C and error bars in G represent ±SEM. Comparisons were done using unpaired two-tailed student’s t-tests, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.