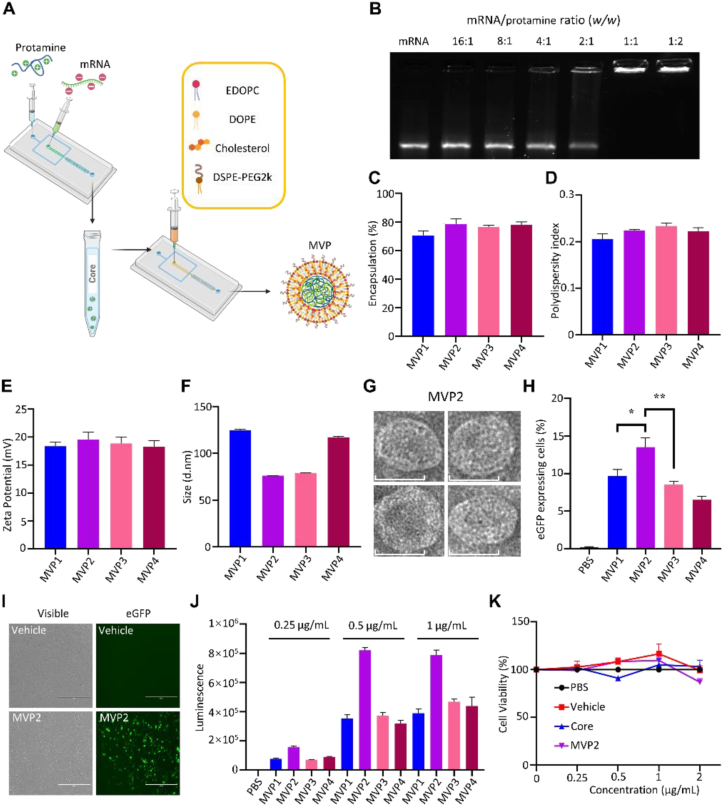
Figure 1.
Preparation and characterization of mRNA particles. (A) Schematic view of vaccine particle preparation. (B) Agarose gel electrophoresis shows that mRNA molecules were retained in the sample-loading well in samples prepared with mRNA/protamine at 1:1 to 1:2 ratio. (C–F) Characterization of mRNA vaccine particles (MVPs) based on percentage of encapsulation, polydispersity index, zeta potential, and size. (G) Representative TEM images of MVP2 particles, scale bar = 50 nm. (H) Percentage of eGFP expression after DC2.4 cells were treated with eGFP-MVPs for 16 h. (I) Fluorescent imaging of eGFP-expressing DC2.4 cells, scale bar = 400 μm. (J) Quantitative analysis on bioluminescence in BMDCs treated with MVPs encapsulated with luciferase-encoding mRNA for 16 h. (K) Changes in the viability of BMDCs treated with PBS, mRNA-free vehicle, mRNA/protamine core, or mRNA-encapsulated MVP2. Treatment concentrations were mRNA-equivalent. Data are presented as mean ± SEM (n = 3). ∗P < 0.05; ∗∗P < 0.01.