Abstract
Background and Hypothesis
Formal thought disorder (FTD) is a core symptom of psychosis, but its neural correlates remain poorly understood. This study tested whether four FTD dimensions differ in their association with brain perfusion and brain structure.
Study Design
This cross-sectional study investigated 110 patients with schizophrenia spectrum disorders using 3T magnetic resonance imaging (MRI). The Thought and Language Disorder scale (TALD) was utilized, which comprises four subscales: Objective Positive (OP), Objective Negative (ON), Subjective Positive (SP), and Subjective Negative (SN). Resting-state cerebral blood flow (rsCBF), cortical thickness (CortTh), gray matter volume (GMV), and diffusion MRI tractography were tested for associations with TALD subscales controlling for age, medication, total intracranial volume, and for variance of the 3 other TALD subscales.
Study Results
Following Bonferroni correction, the FTD dimensions presented distinct neural correlates. OP scores were associated with increased rsCBF and increased GMV in the right cerebellum lingual gyrus. Higher SP scores were linked to increased GMV in bilateral prefrontal cortex. In contrast, ON was associated with increased GMV in the right premotor cortex. At more liberal statistical thresholds, higher SP was associated with increased CortTh in the right inferior frontal gyrus, whereas SN scores were linked to decreased GMV in the right prefrontal lobe, the left inferior temporal gyrus, and the left supplementary motor area. Unadjusted analyses mostly corroborated these findings.
Conclusion
These findings stress the heterogeneity in FTD, suggesting distinct neural patterns for specific FTD experiences. In sum, FTD in psychosis may require distinct treatment strategies and further mechanistic investigations on single-item levels.
Keywords: disorganization, pathobiology, emptiness
Introduction
Formal thought disorder (FTD) is a multidimensional syndrome in many psychiatric disorders that may also occur in healthy subjects. It is a core symptom in schizophrenia with a prevalence of about 50%–80%.1 FTD severity may inform prognosis and predict illness severity, as well as occupational attainment, social functioning, well-being, and the quality of therapeutic relationships.1–7 The heterogeneity of FTD has been described in a positive–negative dichotomy with loose associations and increased speech production on one side and thought blocking and concretism on the opposite.8 Nevertheless, factor analytic studies suggest up to 5 FTD factors, explaining more variance than positive–negative dichotomy.1,9 While the distinction between positive and negative FTD is well established,8 self-reported and clinician’s assessment are rarely distinguished.
Kircher et al.10 introduced a novel rating scale, that is, the thought and language disorder scale (TALD), which distinguishes self-reported (subjective) and clinician reported (objective) as well as positive and negative FTD, resulting in 4 FTD dimensions: objective positive (OP), objective negative (ON), subjective positive (SP), subjective negative (SN). Factor analysis confirmed these 4 FTD dimensions. Two studies linked OP FTD to executive dysfunction, whereas ON FTD was linked to other cognitive impairment and poor functioning, while SN FTD was associated with poor quality of life.11,12 These clinical associations with TALD subscales support the consideration of four distinct FTD dimensions, which are likely to arise from both shared and distinct neural mechanisms. However, little is known on the neural underpinnings of the 4 FTD dimensions.
Structural brain imaging of FTD consistently indicates reduced gray matter volume (GMV) and lower cortical thickness (CorTh) in the language circuitry, including the bilateral superior temporal gyrus (STG), inferior parietal lobe (IPL), the inferior frontal gyrus (IFG), and left middle temporal gyrus (MTG).8,13–17 Furthermore, positive FTD was mainly linked to reduced GMV and lower CorTh in the core cortical language network including bilateral STG and MTG, as well as the left IFG and IPL.17–19 In contrast, negative FTD was associated with reduced GMV or CorTh in frontal cortices and subcortical structures including thalamus and hippocampus.17,19,20 Still, the available data is missing a consistent pattern of gray matter (GM) alterations.
Diffusion tensor imaging (DTI) studies reported white matter (WM) alterations in patients with FTD in major fronto-temporal and fronto-parietal association fibers.19,21–24 The majority of studies indicated increased diffusion in language tracts in patients with severe positive FTD.19,21–23,25
Functional neuroimaging studies on FTD during rest or during language tasks have yielded conflicting findings.8,13 Perfusion magnetic resonance imaging (MRI) studies during the resting-state repeatedly indicated higher perfusion with more severe FTD in regions of the bilateral language network, specifically in the IFG, STG, MTG, and IPL.15,26,27 Few functional MRI (fMRI) studies investigated positive or negative FTD specifically.8 For instance, resting-state perfusion was increased in the STG with higher negative FTD ratings.27 Task fMRI studies indicated lower activity in the left temporal cortex in subjects with increased positive FTD severity.28,29 In contrast, increased negative FTD was linked to higher activity in bilateral dorsolateral prefrontal cortices (DLPFC), inferior frontal, and left temporal cortex during a 2-back task.30
To develop specific treatments for FTD, we need to understand the underlying pathobiology. Studies testing 1 or 2 FTD dimensions broadly indicated an involvement of the language circuitry, but failed to explain specific phenomena while reporting inconsistent patterns of brain-behavior associations. Given the multidimensional structure of FTD, we may need to test four dimensions, including objective, subjective, negative, and positive FTD. As positive and negative FTD typically correlate with each other, any correlation between FTD ratings and a neuroimaging measure will emphasize common over distinct effects of FTD dimensions.8,10,27 Therefore, we will explore the neural correlates of four TALD dimensions utilizing multimodal brain imaging including resting-state perfusion and GM structure in patients with schizophrenia. We hypothesize to replicate the general association of FTD severity with brain structural and perfusion alterations in the broad language network. In addition, we assume distinct patterns of brain-behavior correlations for OP, ON, SP, and SN FTD dimensions. To test specific contributions of each dimension, we will control for covariance with the other dimensions.31
Methods
Participants
For the present study, we collected data from 129 patients, of which 110 entered the final analysis following quality checks (see Supplementary figure S1). We combined data from two clinical trials targeting psychomotor slowing or gesture impairments in schizophrenia spectrum disorders: OCoPS-P (Overcoming Psychomotor Slowing in Psychosis, NCT03921450) and BrAGG-SoS (Brain stimulation And Group therapy to improve Gesture and Social Skills in psychosis, NCT04106427). Recruitment was done at the in- and out-patient departments of the University Hospital of Psychiatry, Bern. All participants provided written informed consent. The study protocols adhered to the declaration of Helsinki and were approved by the local ethics committee. To be included, participants had to be right-handed, age 18–65 years, and to suffer from schizophrenia, schizoaffective disorder, brief psychotic disorder, or schizophreniform disorders according to DSM-5. Exclusion criteria were substance abuse other than nicotine, history of neurologic disease, severe brain injury, transcranial magnetic stimulation in the past 3 months, pregnancy or nursing, and any metal objects in the body. Diagnoses and exclusion criteria were ascertained following clinical diagnostic interviews, physical examination, and review of case files by trained psychiatrists. One hundred four out of 110 patients currently received antipsychotic medication, while 24 (22%) also received additional psychotropic medication, mainly antidepressants (for details see Supplementary table S2). In total 91 had schizophrenia, 14 schizoaffective disorder, and 5 schizophreniform disorder (Supplementary table S4). Twenty-one patients had comorbid diagnoses, including catatonia (14), depression (4), obsessive-compulsive disorder (2), and posttraumatic stress disorder (1).
Procedures
We assessed psychopathology using the TALD10 and the Positive And Negative Syndrome Scale (PANSS).32 The TALD is a comprehensive 30-item clinical rating scale covering a wide array of FTD symptoms including both objectively observable, as well as subjective symptoms in the positive and negative dimensions.10 Thus, the TALD is comprised of four subscales: OP, ON, SP, and SN. The relative score for each subscale was calculated and used in all subsequent analyses, that is, the sum of the subscale divided by the number of items of the subscale (table 1, Supplementary table S1). All raters (LM, AK, DA, DB-G) were clinical psychiatrists, who received TALD training by the principal investigator to achieve optimal inter-rater reliability (α > .80). Psychopathology assessments were performed within 48 h of MRI scanning. We calculated the mean olanzapine equivalents (OLZ eq.) according to Leucht et al.33
Table 1.
Demographic and Clinical Characteristics
| Patients (n = 110) | |
|---|---|
| Demographics | |
| Age (years) | 36.6 ± 12.1 |
| Gender (males %) | 52.7 % |
| Education (years) | 13.5 ± 2.9 |
| Medication (OLZ eq mg) | 15.2 ± 11.0 |
| Duration of illness (years) | 11.9 ± 11.6 |
| Episodes (n) | 7.0 ± 11.2 |
| Assessments | |
| PANSS-total | 69.6 ± 20.7 |
| Positive | 14.6 ± 5.7 |
| Negative | 20.0 ± 7.6 |
| TALD total | 15.8 ± 9.0 |
| Objective positive (relative) | 0.3 ± 0.4 |
| Objective negative (relative) | 1.0 ± 0.9 |
| Subjective positive (relative) | 0.8 ± 0.9 |
| Subjective negative (relative) | 0.9 ± 0.6 |
Note: Values represent the mean ± SD.
Note: OLZ eq, olanzapine equivalent; PANSS, Positive And Negative Syndrome Scale; TALD, Thought and Language Disorder.
MR Imaging
We performed multimodal neuroimaging at the translational imaging center of the Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland and acquired 3 neuroimaging markers: resting-state cerebral blood flow (rsCBF); CortTh, GMV. The MRI scans were acquired on a 3T Prisma MRI whole-body scanner using a 20-channel radio-frequency head coil (Siemens, Germany). Patients lay horizontally in the MR scanner and their arms rested beside their trunks. We placed head motion foam pads around the patients’ heads and we explicitly instructed them to avoid head motion. In addition, in a subgroup of 71 patients, we successfully acquired diffusion-weighted images (DWI). Details regarding the MRI acquisition parameters are given in Supplementary Methods.
Perfusion and Voxel-Based Morphometry Preprocessing.
First, the MP2RAGE T1-3D images were segmented into GM, WM, and cerebrospinal fluid (CSF) using the CAT12 toolbox (http://www.neuro.uni-jena.de/cat/) in SPM12 (Version 7771, Welcome Trust, London, UK. https://www.fil.ion.ucl.ac.uk/spm). The segmented tissues were saved in both native and DARTEL space.
Perfusion Imaging Preprocessing
The pulsed arterial spin labeling preprocessing steps consisted of realignment and unwrapping to correct for motion and distortion due to field inhomogeneity. We then quantified rsCBF (ml/100 g/min) according to a previously applied, standardized protocol34–37 (see Supplementary Methods). CBF Images were realigned and co-registered to the corresponding raw T1-3D image and normalized using the deformation matrix of the corresponding T1-3D image. A denoising step was performed using subject-wise first-level generalized linear models (GLMs) with the WM and CSF signal and the 6 motion parameters from realignment as explanatory variables.37 We used the mean rsCBF map for further analysis. For quality control, we checked six motion parameters (x-, y-, z-translations, roll, pitch, and yaw) and set a limit of 2.5 mm of motion in any direction for exclusion.
Voxel-Based Morphometry
The preprocessing of the GM volume was performed using the DARTEL VBM algorithm with SPM 12, following the standard procedure established by Ashburner.38 First, we created a DARTEL template by integrating the sampled patients’ brains for spatial normalization. Then, the modulated native GM was normalized and smoothed using a 6 mm FHWM Gaussian kernel to the MNI template for position localization using SPM12. Total intracranial volume (TIV) was calculated for each patient by summing GM, WM, and CSF tissue volumes.
CortTh and Diffusion-Weighted Imaging.
Details for the structural imaging preprocessing are given in Supplementary Methods. We used the standard FreeSurfer image analysis suite for cortical reconstruction39 (7.1.1)(http://surfer.nmr.mgh.harvard.edu/) to conduct the preprocessing steps for CortTh analyses. The preprocessing for diffusion-weighted imaging was performed using FSL 6.04 (https://fsl.fmrib.ox.ac.uk/).40,41 For tractograghy, we computed the diffusion tensor parameters (Fractional anisotropy [FA] and mean diffusivity [MD]) for each of the 11 fiber bundles of interest (see Supplementary figure S3), using the Quantitative Imaging Toolkit (QIT,42https://cabeen.io/qitwiki). Tract-Based Spatial Statistics (TBSS) in FSL was used for the voxel-wise statistical analysis of FA and MD.41,43
Statistical Analyses
In all the imaging analyses we controlled for age, medication (OLZ), and TIV. Moreover, when exploring the associations of brain markers with each of the TALD sub-scores (OP, ON, SP, and SN), we entered the other three sub-scores as covariates. Internal consistency of the TALD was tested with Cronbach’s alpha. While all tests were corrected for multiple comparisons, the most stringent correction would include adjusting for 16 tests (4 brain parameters and 4 TALD subscales), yielding a P-value of .0031.
rsCBF Analysis.
For rsCBF analyses, we calculated the association between brain perfusion and FTD severity, using a second-level multiple regression model for TALD total score and another for the TALD sub-scores. We set a cluster-forming threshold of P = .005 and a P-value corrected with false discovery rate (qFDR) corrected < .05 for the cluster-wise threshold.
CortTh Analysis.
To identify the association between CortTh and TALD total or TALD sub-scores, group comparisons were performed using the GLM and DODS (“different offset, different slope”) method with no smoothing.44 Left and right hemispheres were analyzed separately. The main results were corrected for multiple comparisons with the standard process, that is, a pre-cached cluster-wise Monte-Carlo simulation using a cluster-wise forming threshold of P < .05 and 10 000 random permutations.45 Statistical models were also corrected for the 2 hemispheres, respectively, and the cluster-level P-value was set at .02546 to correct for multiple comparisons. Furthermore, the CortTh value of the significant cluster provided by Freesurfer was exported to run a partial correlation (Kendall method) in R (version 4.0.2) using the ppcor package for the TALD sub-scores and added age, medication, TIV, and the other TALD sub-scores as covariates of non-interest.
VBM Analysis.
For VBM analysis, we used an absolute threshold of.1 to ensure the inclusion of GM voxels with a probability ≥ .1 of being GM. To evaluate the association between local GMV and FTD severity, we used a second-level multiple regression model for TALD total score and a second one for the TALD sub-scores. We set a cluster-forming threshold of P = .005 and a P-value qFDR corrected < .05 for the cluster-wise threshold.
DTI Analyses.
These exploratory analyses were performed only in a subset of 71 patients. Using the preprocessed DWI, we performed tractography analyses as well as TBSS to explore the association between the measures of structural connectivity and FTD severity. For tractography analysis, Kendall tau correlation analyses were performed between the mean FA of each of the 11 fiber bundles and TALD total score, as well as the TALD sub-scores. We performed the same set of analyses on the MD values. A P-value < .05 FDR corrected was considered statistically significant for these exploratory analyses. Within TBSS, a regression analysis of whole-brain FA and MD with TALD total and subscales was performed. The level of significance was set at P-FWE corrected < .05, using a threshold-free cluster enhancement with 5000 randomized permutations.
Results
Demographic and Clinical Characteristics
Clinical and demographic characteristics of the 110 patients are presented in table 1. Symptom severity as measured by the PANSS was moderate to severe, as was FTD according to the TALD, but no gender differences or differences between diagnoses appeared (see Supplementary tables S3 and S4). Using Kendall’s tau we tested bivariate correlations between the 4 TALD subscales across subjects. The subjective subscales (SP and SN), the positive subscales (OP and SP), as well as the negative subscales (ON and SN) showed positive correlations (Supplementary figure S2). Internal consistency of the TALD and its subscales was acceptable to good (TALD total α = .77, SP α = .56, SN α = .70, OP α = .83, ON α = .67).
General FTD Severity—TALD Total
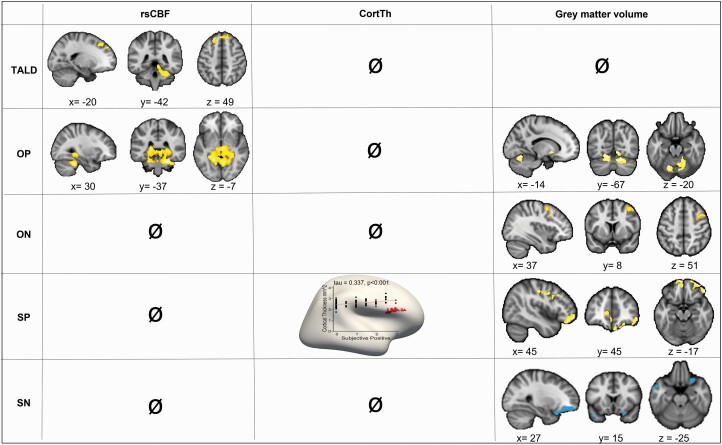
The multiple regression model exploring the association between TALD and whole-brain rsCBF indicated that higher FTD severity is associated with increased rsCBF in bilateral DLPFC (Broadman area [BA] 9), left frontal eye field (BA 8), and right cerebellum (figure 1, table 2). Neither for CorTh nor for GMV did we observe associations with the TALD total score.
Fig. 1.
Associations of TALD total score, as well as TALD sub-scores (OP, ON, SP, SN) severity with rsCBF, CorthTh, and GMV analyses corrected for each other subscale, age, medication (OLZ eq), and TIV. Red/yellow denotes a positive association (corrected P-value < .05), while blue denotes a negative association (corrected P-value < .05). Specific statistics of significant clusters are given in table 2. Detailed associations of TALD sub-scores with GMV are presented in Supplementary figure 5. TALD, Thought and Language Disorder scale; OP, objective positive, ON, objective negative; SP, subjective positive, SN, subjective negative; rsCBF, resting-state cerebral blood flow; CortTh, cortical thickness; GMV, gray matter volume; OLZ eq, olanzapine equivalents..
Table 2.
MRI Analysis Summary of Only Significant Clusters for the Associations of TALD Total Score, As well as, TALD Sub-scores (OP, ON, SP, SN) Severity for rsCBF, CorthTh, and GMV Analyses
| Cluster Size | pFWE-corr | pFDR-corr | Coordinates | Brain Area | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| TALD | |||||||
| rsCBF | 803 | .007 | .006 | 3 | 53 | 37 | R. DLPFC (BA 9) |
| −17 | 47 | 38 | L. DLPFC (BA9) | ||||
| −20 | 32 | 48 | L. Frontal eye field (BA8) | ||||
| 950 | .003 | .005 | 20 | −35 | −31 | R. cerebellum (Lobule V) | |
| 10 | −66 | ‐12 | R. cerebellum (Lobule VI) | ||||
| 35 | −39 | −31 | R. cerebellum (Lobule VI) | ||||
| CortTh | ø | ||||||
| GMV | ø | ||||||
| OP | |||||||
| rsCBF | 5892 | <.001 | <.001* | 24 | −36 | −29 | Bilateral cerebellum (Lobule I–IV) Bilateral parahippocampus |
| 35 | −41 | −25 | Bilateral fusiform gyrus (BA 37) | ||||
| 6 | −20 | −3 | Bilateral thalamus | ||||
| CortTh | Ø | ||||||
| GMV | 1968 | .001 | .001* | 11 | −69 | −8 | R. Lingual Gyrus |
| 20 | −66 | −24 | R. Cerebellum (Lobule VI) | ||||
| 823 | .089 | .032 | −17 | 5 | −6 | L. Putamen | |
| 817 | .091 | .032 | −17 | −68 | −23 | L. Cerebellum (Lobule VI) | |
| ON | |||||||
| rsCBF | Ø | ||||||
| CortTh | Ø | ||||||
| GMV | 753 | .003 (peak) | .002 (peak)* | 42 | 15 | 41 | R. Middle Frontal Gyrus |
| SP | |||||||
| rsCBF | Ø | ||||||
| CortTh | 336 | <.05 | 54 | 29 | 12 | R. Pars triangularis | |
| GMV | 3845 | < .001 | <.001* | 50 | 38 | −17 | R. Pars orbitalis (BA 47) |
| −11 | 53 | 8 | L. Ant.Prefrontal Cortex(BA 10) | ||||
| −8 | 65 | −15 | L. Orbito-Frontal (BA 11) | ||||
| 920 | .056 | .084 | 50 | 12 | 33 | R. Broca-Operculum (BA 44) | |
| 56 | −6 | 44 | R. Premotor + SMA (BA 6) | ||||
| 44 | −18 | 42 | R. Primary Motor Cortex (BA 4) | ||||
| SN | |||||||
| rsCBF | Ø | ||||||
| CortTh | Ø | ||||||
| GMV | 992 | .041 | .048 | −41 | −2 | 44 | L. Premotor + SMA (BA 6) |
| −39 | 9 | ‐42 | L. Inferior temporal Gyrus (BA 20) | ||||
| −48 | 6 | ‐36 | L. Temporal Pole (BA 38) | ||||
| 908 | .068 | .048 | 26 | 45 | ‐9 | L. Ant. Prefrontal Cortex (BA 10) | |
| 41 | 30 | −14 | R. Pars orbitalis (BA 47) | ||||
*Indicates P-values that would survive additional correction for 16 tests (P < .0031).
FTD Dimensions—TALD Subscales
Objective Positive
The multiple regression model exploring the association between OP sub-score and whole-brain rsCBF showed that higher OP scores were associated with increased rsCBF in bilateral cerebellum, bilateral thalamus, and bilateral limbic areas (figure 1, table 2). Furthermore, we observed OP severity to be associated with higher GM volume in the bilateral cerebellum as well as in the right putamen and lingual gyrus (figure 1, table 2, Supplementary figure S4). However, OP sub-score and whole-brain CortTh were not associated.
Objective Negative
The model exploring the ON sub-score showed no association with whole-brain rsCBF. Likewise, we found no correlation with whole-brain CortTh. In contrast, ON severity was associated with higher GMV in the right middle frontal gyrus including the right supplementary motor area (SMA) (figure 1, table 2, Supplementary figure S4).
Subjective Positive (SP)
Exploring the association between SP and whole-brain CortTh, we observed that higher SP severity was linked to increased CortTh in the right IFG pars triangularis. Furthermore, SP severity was associated with higher GMV in the bilateral anterior prefrontal cortex, right orbital part of the IFG, as well as left orbito-frontal cortex. In addition, trend associations between SP severity and higher GMV were noted in right primary motor cortex, SMA, and right Broca’s area (figure 1, table 2, Supplementary figure S4). However, we found no association with whole-brain rsCBF.
Subjective Negative
SN severity was associated with lower GMV in the right anterior prefrontal cortex and right orbital part of the IFG as well as left premotor cortex and SMA, left inferior temporal gyrus, and left temporal pole (figure 1, table 2, Supplementary figure S4). However, no associations were detected between SN scores and CBF or CortTh.
Unadjusted TALD Subscales
When we analyzed the associations of the TALD subscales without correcting for the other subscales, results remained very similar (Supplementary figure 6). However, we found increased SN to be linked to increased rsCBF in left prefrontal and right premotor cortex, while its association with lower GMV disappeared.
Exploratory Analyses of DTI Data
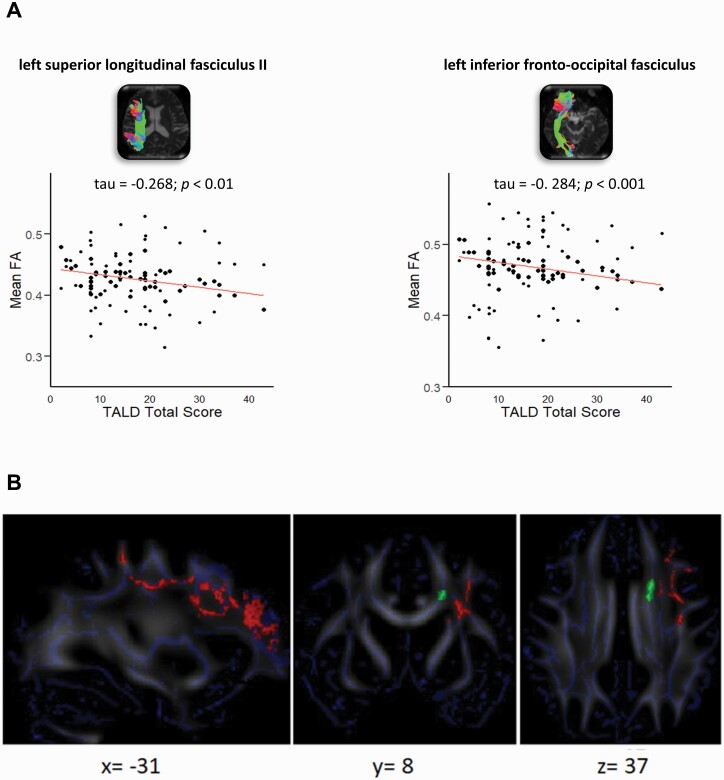
In the tractography analyses, only the TALD total score showed negative correlations (table 3) with mean FA within left and right inferior fronto-occipital fasciculus (IFOF), left superior longitudinal fasciculus II, right uncinate fasciculus, and superior temporal cortex connections via the corpus callosum. As an example, 2 correlation plots are presented in figure 2A. No statistically significant association was observed for MD or any TALD sub-scores.
Table 3.
Partial Correlation of the Association Between Total TALD Severity and Mean FA and MD Values (Corrected for Age, Medication [OLZ eq] and TIV) for a Subset of Our Sample Size (n = 71) for 11 Bundles Following Diffusion Tensor Imaging Analyses
| FA | MD | |||||
|---|---|---|---|---|---|---|
| tau | P-value (unc) | P-value (fdr) | tau | P-value (unc) | P-value (fdr) | |
| UNCr | −.222 | .007** | .015* | .168 | .044* | .242 |
| IFOFr | −.242 | .003** | .015* | .135 | .105 | .288 |
| SCPr | .099 | .231 | .290 | .020 | .803 | .896 |
| ICPr | .081 | .326 | .358 | .082 | .324 | .509 |
| SLFIIr | −.097 | .238 | .290 | .098 | .238 | .436 |
| CC | −.268 | .007** | .015* | .083 | .409 | .562 |
| UNCl | −.154 | .062 | .113 | .122 | .144 | .316 |
| IFOFl | −.284 | .0006** | .006** | .135 | .105 | .288 |
| SCPl | −.010 | .897 | .897 | .010 | .898 | .898 |
| ICPl | .101 | .226 | .290 | .019 | .815 | .896 |
| SLFIIl | −.268 | .007** | .015* | .205 | .014* | .154 |
Note: UNCr, right uncinate fasciculus; IFOFr, right inferior fronto-occipital fasciculus; SCPr, right superior cerebellar peduncle; ICPr, right inferior cerebellar peduncle; SLFIIr, right superior longitudinal fasciculus II (connections to middle frontal cortex); CC, corpus callosum connecting prefrontal cortex to temporal pole and superior temporal cortex; UNCl, left uncinate fasciculus; IFOFl, left inferior fronto-occipital fasciculus; SCPl, left superior cerebellar peduncle; ICPl, left inferior cerebellar peduncle; SLFIIl, left superior longitudinal fasciculus II (connections to middle frontal cortex).
*Denotes a significant association P-value < .05.
**Denotes a significant association P-value < .01.
Fig. 2.
(A) Example correlation plots for 2 bundles (superior longitudinal fasciculus II and left inferior frontal-occipital fasciculus) showing the association between mean FA and total TALD score. (B) TBSS results. Significant association between SP TALD sub-score severity and MD (red [in print: dark grey], TFCE FWE P = .045, 5000 simulations) and at trend level FA (green [in print: light grey], TFCE FWE P = .09) when controlling for age, medication (OLZ eq), TIV, and the other sub-scores. Blue = Skeleton. TALD, Thought and Language Disorder scale; SP, subjective positive; MD, mean diffusivity; TBSS, Tract-Based Spatial Statistics; OLZ eq, olanzapine equivalents..
In the TBSS analysis, the multiple regression model exploring the association between SP severity and MD demonstrated a significant positive association within the frontal lobe (cluster: 2062 voxels, threshold free cluster enhancement [TFCE] p-value corrected with family-wise error [qFWE] P = .045) in cognitive, motor, and language-associated pathways (figure 2B). This sub-score severity also presented a trend for a positive association with mean FA in the frontal lobe (cluster: 94 voxels, TFCE qFWE P = .09) in the interhemispheric premotor pathway. All other associations were not statistically significant.
Discussion
In 110 patients with schizophrenia spectrum disorders, we aimed at exploring the neural correlates of four distinct dimensions of FTD using perfusion and structural MRI. In line with our hypotheses, we found distinct associations for each of the 4 FTD dimensions controlling for the severity of the other FTD dimensions. Four main findings emerged: (1) OP FTD was associated with increased resting-state perfusion and GMV in subcortical areas, such as bilateral cerebellum, thalamus, and basal ganglia. (2) SP FTD was associated with increased GMV and CorTh in the right IFG, whereas (3) SN FTD was linked to reduced GMV in premotor cortex and IFG. And (4) ON in turn was associated with increased GMV in premotor cortices.
Language is processed by a left-lateralized fronto-temporal association network, including Broca’s and Wernicke’s regions as well as the arcuate fascicle.47–49 In addition, this language network is closely tied to other important brain networks, such as the default mode, salience, and central executive networks.48 Components of the network are specialized, for example, superior and middle temporal lobe for semantic memory, IFG for integrating multiple information, and the cingulate and DLPFC for language control, for example, discourse planning. Brain alterations located in the core language network and adjunct cortical and subcortical brain areas are neural correlates of the clinical phenomenon of FTD.8,47,48 In line with previous studies on FTD, the present study linked FTD severity to neural alterations in orbito-frontal and medialfrontal cortices, thalamus, and cerebellum.8,14,16,20 Indeed, as in previous reports, both negative FTD dimensions (ON and SN) were associated with bilateral GM alterations in orbito-frontal and medialfrontal cortices.8,14,16,17,20 Similarly, the current findings on resting-state perfusion broadly corroborate previous reports on altered perfusion and task-based activation in the cerebellum and medial temporal lobe in patients with FTD.8,27,50
Besides these commonalities, this study extends previous knowledge by applying multimodal brain imaging and a multidimensional FTD measure that also includes subjective FTD. Differences between our study and prior reports are likely to arise from three important factors: sample characteristics, FTD measure used, and neuroimaging analyses applied. Indeed, sample size and heterogeneous clinical characteristics heavily impact associations between brain and behaviors. Our patients had a broad distribution of FTD severity, general symptom severity, and chronicity. While previous reports applied the Thought, Language, and Communication scale, our study used the TALD covering FTD much broader allowing to explore 4 distinct FTD dimensions, including 2 on the subjective experience of FTD.8,10,47,50,51 Finally, we chose to explore the contribution of each dimension separately, correcting for the variance of the other three dimensions. While this approach enables to test associations specific to single FTD dimensions, it is also prone to eliminate the effect of common pathways to FTD as their scores jointly correlate. The FTD dimensions are not mutually exclusive in the single subject, instead, some participants score on more than one FTD dimension. Thus, with correction for the other dimensions, shared neural alterations among the subscales are excluded in these results. However, when we explored the associations without correcting for the other subscales, results generally included clusters of similar size and location, suggesting small contributions of the shared variance among FTD dimensions. Only in the SN dimension did results change with the novel appearance of rsCBF clusters and GMV clusters vanishing, which might be due to strong correlations of SN with SP and ON. Collectively, the current results should be appreciated as complementary to previous findings.
Resting-state CBF is a marker of local metabolic activity. Previous studies reported more severe FTD to be associated with higher rsCBF in the core language network, i.e., bilateral STG, angular gyrus, or left IFG.15,27,52 Our study found higher TALD total scores (severer FTD) to be linked to increased rsCBF in adjunct regions of the language network, for example in bilateral DLPFC and right cerebellum. The DLPFC has been proposed to critically contribute to language processing, for example when processing syntax, prosody, coherence.53 Likewise, the cerebellum is involved in word generation, verbal working memory, and articulatory movements.54–56 Thus, increased neural activity in bilateral DLPFC might reflect specific pathology causing defective language control, such as peculiar articulation or loose associations. Likewise, OP severity was linked to increased rsCBF in thalamus, fusiform gyrus, and cerebellum. Indeed, increased neural activity during increased speech production, tangentiality, or neologisms seems plausible. For example, patients with positive FTD had increased neural activity in cerebellum, fusiform gyrus, and caudate in fMRI-tasks of free speech.29,57,58
In general, FTD severity has previously been linked to reduced GMV and lower CorTh in the language circuitry, including the bilateral STG, IPL, IFG, and left MTG.8,13–17 In line with our hypotheses, we found 3 FTD dimensions (OP, ON, SP) to be associated with increased GMV, while 1 (SN) was linked to reduced GMV.
The OP subscale summarizes observable signs of increased thought and language production, for example, logorrhea, pressured speech, and neologisms.10 OP severity was associated with increased GMV in right putamen and bilateral cerebellum, where we noted spatial overlap with findings of increased rsCBF. The association of GMV and OP severity was detected in bilateral lobule VI, an area active during verb generation, motor verbal utterance, and verbal working memory.54,56 Collectively, observable positive FTD seems to be associated with speech motor regions.
The SP subscale includes the subjective experiences of pressure of thought and thought interference.10 Increased SP scores were specifically associated with altered GM in the executive network, which is thought to control language output and often found less efficient in subjects with conceptual disorganization.48
The ON subscale represents qualitative and quantitative reductions of language and thought with items such as concretism and poverty of speech.10 Higher ON scores were associated with increased GMV premotor areas. A similar effect had previously been reported for reduced motor behavior in schizophrenia, which was associated with increased GMV and increased rsCBF in the SMA.35,59
The SN subscale includes items such as poverty of thought, rumination, or blocking.10 SN was the only subscale linked to decreased GMV in multiple fronto-temporal areas including the SMA. The anterior prefrontal cortex is critical in planning and allocating resources of attention.60 Disturbance of these functions could give rise to typical features of SN, such as inhibited thinking, or impaired speech reception. SN was also associated with decreased GMV in the left temporal pole, which serves important semantic functions such as naming, word-object labeling, and semantic processing in all modalities.61
Our exploratory diffusion tensor imaging tractography analysis detected higher total TALD scores to be associated with reduced FA, indicating more diffusion and less organized WM microstructure, in the ventral language stream (left and right IFOF, right uncinate fasciculus). These results are in line with most reports linking FTD to FA in major fronto-temporal and fronto-parietal association fibers.19,21–25
While some cognitive domains and clinical courses have been linked to sex differences, this study did not find sex differences in the clinical presentation of FTD.62,63 Also, the reported neuroimaging correlates were unchanged when adding sex as covariate to the models (data not shown).
This study has several limitations. First, we exclusively focused on FTD in the context of schizophrenia spectrum disorders. Thus, our results may not translate to FTD in other conditions. Second, the analyses combined data from two RCTs introducing potential selection bias, as patients consented to complicated procedures. Nevertheless, subjects had substantial illness severity and duration. Third, 104 out of 110 patients received antipsychotic medication at the time of the study. As antipsychotics may affect both brain structure and FTD severity, we covaried for current dosage in all analyses. In addition, we corrected imaging analyses for TIV and age. Still, in the small group of patients with other medication, further effects on brain structure are possible. Fourth, DTI was available only in a subset of our sample (n = 71), limiting the statistical power for these analyses. Fifth, comparisons across imaging modalities are hampered by the application of different techniques to control for multiple comparisons, however, we chose the standard corrections for each neuroimaging method. Sixth, the cross-sectional design of the study does not allow inferences on whether rsCBF increases may compensate for altered GMV.14 Finally, the TALD subscales include heterogeneous numbers of items (OP > SN > ON > SP) due to the origin of factor analysis. We calculated a relative score for each subscale, but the variance explained by the total TALD differs between subscales, rendering some subscales more robust than others.10
Conclusion
This study found distinct neural correlates across multiple MRI measures for four FTD dimensions in psychosis, extending prior work on positive and negative FTD. Cerebellar structure and perfusion are implicated in OP FTD, IFG in SP FTD, and premotor cortex volumes in objective and SN FTD. Findings suggest that specific FTD dimensions may be targeted by non-invasive brain stimulation, calling for more refined investigations on FTD networks, as well as longitudinal and transdiagnostic studies.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Contributor Information
Lydia Maderthaner, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Anastasia Pavlidou, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Stephanie Lefebvre, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Niluja Nadesalingam, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Victoria Chapellier, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Sofie von Känel, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Alexandra Kyrou, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Danai Alexaki, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Florian Wüthrich, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Florian Weiss, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Daniel Baumann-Gama, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Roland Wiest, Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland; Institute of Diagnostic and Interventional Neuroradiology, Inselspital, University of Bern, Bern, Switzerland.
Werner Strik, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Tilo Kircher, Department of Psychiatry and Psychotherapy, University of Marburg, Marburg, Germany.
Sebastian Walther, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Funding
This study received funding from the Swiss National Science Foundation grants #182469 and #184717 to SW. The funder had no influence on the data, analyses, or decision to publish.
Conflict of Interest
Sebastian Walther received honoraria from Janssen, Lundbeck, Mepha, Neurolite, and Sunovion, which are unrelated to this study. All other authors reported no potential conflicts of interest.
References
- 1. Roche E, Creed L, MacMahon D, Brennan D, Clarke M.. The epidemiology and associated phenomenology of formal thought disorder: a systematic review. Schizophr Bull. 2015;41(4):951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cavelti M, Homan P, Vauth R.. The impact of thought disorder on therapeutic alliance and personal recovery in schizophrenia and schizoaffective disorder: an exploratory study. Psychiatry Res. 2016;239:92–98. [DOI] [PubMed] [Google Scholar]
- 3. Sigaudo M, Crivelli B, Castagna F, et al. Quality of life in stable schizophrenia: the relative contributions of disorganization and cognitive dysfunction. Schizophr Res. 2014;153(1–3):196–203. [DOI] [PubMed] [Google Scholar]
- 4. Tan EJ, Thomas N, Rossell SL.. Speech disturbances and quality of life in schizophrenia: differential impacts on functioning and life satisfaction. . Compr Psychiatry. 2014;55(3):693–698. [DOI] [PubMed] [Google Scholar]
- 5. Marggraf MP, Lysaker PH, Salyers MP, Minor KS.. The link between formal thought disorder and social functioning in schizophrenia: a meta-analysis. Eur Psychiatry. 2020;63(1):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Comparelli A, Corigliano V, Forcina F, et al. The complex relationship among formal thought disorders, neurocognition, and functioning in nonacutely ill schizophrenia patients. J Nerv Ment Dis. 2020;208(1):48–55. [DOI] [PubMed] [Google Scholar]
- 7. Oeztuerk OF, Pigoni A, Wenzel J, et al. The clinical relevance of formal thought disorder in the early stages of psychosis: results from the PRONIA study. Eur Arch Psychiatry Clin Neurosci. 2022;272(3):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kircher T, Brohl H, Meier F, Engelen J.. Formal thought disorders: from phenomenology to neurobiology. Lancet Psychiatry. 2018;5(6):515–526. [DOI] [PubMed] [Google Scholar]
- 9. Yalincetin B, Bora E, Binbay T, Ulas H, Akdede BB, Alptekin K.. Formal thought disorder in schizophrenia and bipolar disorder: a systematic review and meta-analysis. Schizophr Res. 2017;185:2–8. [DOI] [PubMed] [Google Scholar]
- 10. Kircher T, Krug A, Stratmann M, et al. A rating scale for the assessment of objective and subjective formal Thought and Language Disorder (TALD). Schizophr Res. 2014;160(1):216–221. [DOI] [PubMed] [Google Scholar]
- 11. Nagels A, Fahrmann P, Stratmann M, et al. Distinct neuropsychological correlates in positive and negative formal thought disorder syndromes: the Thought and Language Disorder Scale in endogenous psychoses. Neuropsychobiology. 2016;73(3):139–147. [DOI] [PubMed] [Google Scholar]
- 12. Mutlu E, Abaoğlu H, Barışkın E, et al. The cognitive aspect of formal thought disorder and its relationship with global social functioning and the quality of life in schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2021;56(8):1399–1410. [DOI] [PubMed] [Google Scholar]
- 13. Cavelti M, Kircher T, Nagels A, Strik W, Homan P.. Is formal thought disorder in schizophrenia related to structural and functional aberrations in the language network? A systematic review of neuroimaging findings. Schizophr Res. 2018;199:2–16. [DOI] [PubMed] [Google Scholar]
- 14. Sumner PJ, Bell IH, Rossell SL.. A systematic review of the structural neuroimaging correlates of thought disorder. Neurosci Biobehav Rev. 2018;84:299–315. [DOI] [PubMed] [Google Scholar]
- 15. Horn H, Federspiel A, Wirth M, et al. Structural and metabolic changes in language areas linked to formal thought disorder. Br J Psychiatry. 2009;194(2):130–138. [DOI] [PubMed] [Google Scholar]
- 16. Horn H, Federspiel A, Wirth M, et al. Gray matter volume differences specific to formal thought disorder in schizophrenia. Psychiatry Res. 2010;182(2):183–186. [DOI] [PubMed] [Google Scholar]
- 17. Sans-Sansa B, McKenna PJ, Canales-Rodríguez EJ, et al. Association of formal thought disorder in schizophrenia with structural brain abnormalities in language-related cortical regions. Schizophr Res. 2013;146(1):308–313. [DOI] [PubMed] [Google Scholar]
- 18. Palaniyappan L, Al-Radaideh A, Gowland PA, Liddle PF.. Cortical thickness and formal thought disorder in schizophrenia: an ultra high-field network-based morphometry study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;101:109911. [DOI] [PubMed] [Google Scholar]
- 19. Stein F, Buckenmayer E, Brosch K, et al. Dimensions of formal thought disorder and their relation to gray- and white matter brain structure in affective and psychotic disorders. Schizophr Bull. 2022;48(4):902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palaniyappan L, Mahmood J, Balain V, Mougin O, Gowland PA, Liddle PF.. Structural correlates of formal thought disorder in schizophrenia: an ultra-high field multivariate morphometry study. Schizophr Res. 2015;168(1–2):305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cavelti M, Winkelbeiner S, Federspiel A, et al. Formal thought disorder is related to aberrations in language-related white matter tracts in patients with schizophrenia. Psychiatry Res Neuroimaging. 2018;279:40–50. [DOI] [PubMed] [Google Scholar]
- 22. Viher PV, Stegmayer K, Giezendanner S, et al. White matter correlates of the disorganized speech dimension in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2018;268(1):99–104. [DOI] [PubMed] [Google Scholar]
- 23. Bopp MHA, Zöllner R, Jansen A, Dietsche B, Krug A, Kircher TTJ.. White matter integrity and symptom dimensions of schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2017;184:59–68. [DOI] [PubMed] [Google Scholar]
- 24. Asami T, Saito Y, Whitford TJ, et al. Abnormalities of middle longitudinal fascicle and disorganization in patients with schizophrenia. Schizophr Res. 2013;143(2–3):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Surbeck W, Hanggi J, Scholtes F, et al. Anatomical integrity within the inferior fronto-occipital fasciculus and semantic processing deficits in schizophrenia spectrum disorders. Schizophr Res. 2020;218:267–275. [DOI] [PubMed] [Google Scholar]
- 26. Stegmayer K, Strik W, Federspiel A, Wiest R, Bohlhalter S, Walther S.. Specific cerebral perfusion patterns in three schizophrenia symptom dimensions. Schizophr Res. 2017;190:96–101. [DOI] [PubMed] [Google Scholar]
- 27. Stegmayer K, Stettler M, Strik W, et al. Resting state perfusion in the language network is linked to formal thought disorder and poor functional outcome in schizophrenia. Acta Psychiatr Scand. 2017;136(5):506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kircher T, Whitney C, Krings T, Huber W, Weis S.. Hippocampal dysfunction during free word association in male patients with schizophrenia. Schizophr Res. 2008;101(1–3):242–255. [DOI] [PubMed] [Google Scholar]
- 29. Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK.. Neural correlates of formal thought disorder in schizophrenia: preliminary findings from a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(8):769–774. [DOI] [PubMed] [Google Scholar]
- 30. Fuentes-Claramonte P, López-Araquistain L, Sarró S, et al. Brain functional correlates of formal thought disorder in schizophrenia: examining the frontal/dysexecutive hypothesis. Psychol Med. 2021;51(14):2446–2453. [DOI] [PubMed] [Google Scholar]
- 31. Kircher T. Neurobiological foundations of thought and language disorder in schizophrenia. Fortschr Neurol Psychiatr. 2008;76(Suppl 1):S24–S32. [DOI] [PubMed] [Google Scholar]
- 32. Kay SR, Fiszbein A, Opler LA.. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 33. Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cantisani A, Stegmayer K, Bracht T, et al. Distinct resting-state perfusion patterns underlie psychomotor retardation in unipolar vs. bipolar depression. Acta Psychiatr Scand. 2016;134(4):329–338. [DOI] [PubMed] [Google Scholar]
- 35. Walther S, Schappi L, Federspiel A, et al. Resting-state hyperperfusion of the supplementary motor area in Catatonia. Schizophr Bull. 2017;43(5):972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA.. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003;49(5):796–802. [DOI] [PubMed] [Google Scholar]
- 37. Muller M, Wuthrich F, Federspiel A, et al. Altered central pain processing in fibromyalgia-a multimodal neuroimaging case-control study using arterial spin labelling. PLoS One. 2021;16(2):e0235879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. 2009;27(8):1163–1174. [DOI] [PubMed] [Google Scholar]
- 39. Dale AM, Fischl B, Sereno MI.. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 40. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM.. FSL. NeuroImage. 2012;62(2):782–790. [DOI] [PubMed] [Google Scholar]
- 41. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 42. Cabeen RP, Laidlaw DH, Toga AW.. Quantitative Imaging Toolkit: software for interactive 3D. Visualization, processing, and analysis of neuroimaging datasets. Paper presented at: International Society for Magnetic Resonance in Medicine (ISMRM), Paris, France; June 16–21; 2018. [Google Scholar]
- 43. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 44. Marie D, Maingault S, Crivello F, Mazoyer B, Tzourio-Mazoyer N.. Surface-based morphometry of cortical thickness and surface area associated with Heschl’s Gyri duplications in 430 healthy volunteers. Front Hum Neurosci. 2016;10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hagler DJ, Jr., Saygin AP, Sereno MI.. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33(4):1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ni HC, Lin HY, Chen YC, Tseng WI, Gau SS.. Boys with autism spectrum disorder have distinct cortical folding patterns underpinning impaired self-regulation: a surface-based morphometry study. Brain Imaging Behav. 2020;14(6):2464–2476. [DOI] [PubMed] [Google Scholar]
- 47. Strik W, Stegmayer K, Walther S, Dierks T.. Systems neuroscience of psychosis: mapping schizophrenia symptoms onto brain systems. Neuropsychobiology. 2017;75(3):100–116. [DOI] [PubMed] [Google Scholar]
- 48. Palaniyappan L. Dissecting the neurobiology of linguistic disorganisation and impoverishment in schizophrenia. Semin Cell Dev Biol. 2022;129:47–60. [DOI] [PubMed] [Google Scholar]
- 49. Braga RM, DiNicola LM, Becker HC, Buckner RL.. Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks. J Neurophysiol. 2020;124(5):1415–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wensing T, Cieslik EC, Müller VI, Hoffstaedter F, Eickhoff SB, Nickl-Jockschat T.. Neural correlates of formal thought disorder: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2017;38(10):4946–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andreasen NC. Scale for the assessment of thought, language, and communication (TLC). Schizophr Bull. 1986;12(3):473–482. [DOI] [PubMed] [Google Scholar]
- 52. Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS.. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. [DOI] [PubMed] [Google Scholar]
- 53. Hertrich I, Dietrich S, Blum C, Ackermann H.. The role of the dorsolateral prefrontal cortex for speech and language processing. Front Hum Neurosci. 2021;15:645209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ashida R, Cerminara NL, Edwards RJ, Apps R, Brooks JCW.. Sensorimotor, language, and working memory representation within the human cerebellum. Hum Brain Mapp. 2019;40(16):4732–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murdoch BE. The cerebellum and language: historical perspective and review. Cortex. 2010;46(7):858–868. [DOI] [PubMed] [Google Scholar]
- 56. Schmahmann JD, Weilburg JB, Sherman JC.. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6(3):254–267. [DOI] [PubMed] [Google Scholar]
- 57. Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK.. Reversed lateralization of temporal activation during speech production in thought disordered patients with schizophrenia. Psychol Med. 2002;32(3):439–449. [DOI] [PubMed] [Google Scholar]
- 58. McGuire PK, Quested DJ, Spence SA, Murray RM, Frith CD, Liddle PF.. Pathophysiology of “positive” thought disorder in schizophrenia. Br J Psychiatry. 1998;173:231–235. [DOI] [PubMed] [Google Scholar]
- 59. Stegmayer K, Horn H, Federspiel A, et al. Supplementary motor area (SMA) volume is associated with psychotic aberrant motor behaviour of patients with schizophrenia. Psychiatry Res. 2014;223(1):49–51. [DOI] [PubMed] [Google Scholar]
- 60. Ramnani N, Owen AM.. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5(3):184–194. [DOI] [PubMed] [Google Scholar]
- 61. Herlin B, Navarro V, Dupont S.. The temporal pole: From anatomy to function—a literature appraisal. J Chem Neuroanat. 2021;113:101925. [DOI] [PubMed] [Google Scholar]
- 62. Leger M, Neill JC.. A systematic review comparing sex differences in cognitive function in schizophrenia and in rodent models for schizophrenia, implications for improved therapeutic strategies. Neurosci Biobehav Rev. 2016;68:979–1000. [DOI] [PubMed] [Google Scholar]
- 63. Sommer IE, Tiihonen J, van Mourik A, Tanskanen A, Taipale H.. The clinical course of schizophrenia in women and men—a nation-wide cohort study. Npj Schizophr. 2020;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.