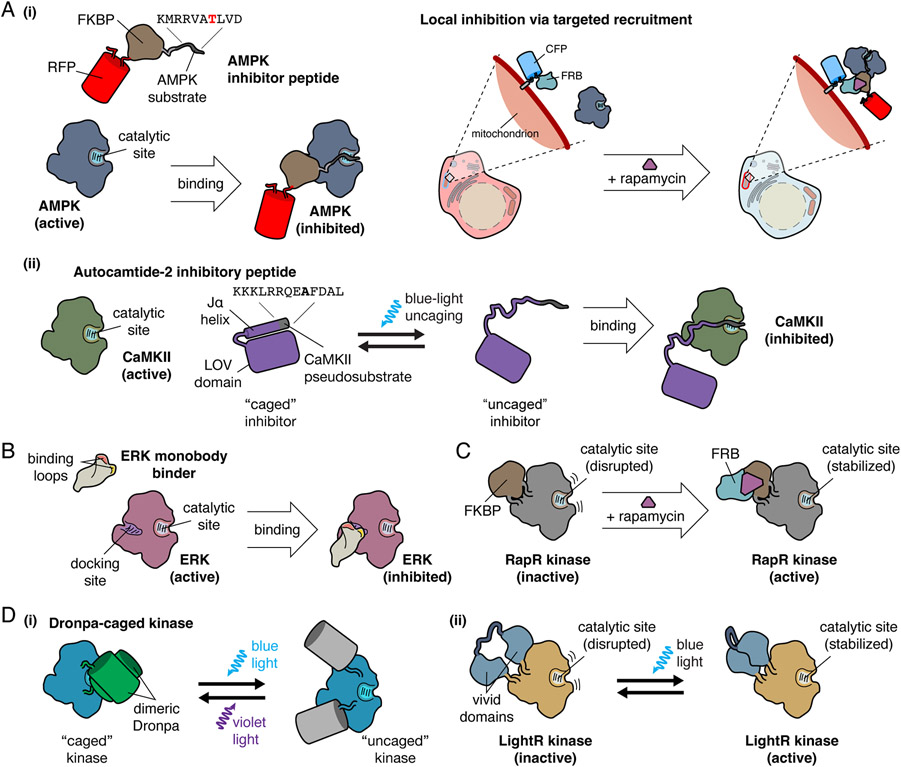
Figure 1. Design of Genetically Encodable Molecular Tools.
A, Examples of competitive kinase inhibitory peptides. (i) AMPK inhibitory peptide is a substrate that binds to the active site of AMPK, functioning as a competitive inhibitor. Local inhibition of AMPK is accomplished through fusion of AMPK inhibitory peptide to FKBP and a fluorescent protein for visualization. CFP-FRB targeted to the mitochondria recruits the FKBP-fused inhibitor to the mitochondria upon the addition of rapamycin, resulting in targeted inhibition of mitochondrial AMPK activity. (ii) A CaMKII pseudosubstrate, termed autocamtide-2 inhibitory peptide, binds to the catalytic site of CaMKII, and functions as a competitive inhibitor. For temporal inhibition of CaMKII, autocamtide-2 inhibitory peptide was fused to the Jα helix of the LOV domain, which is caged in the absence of blue light. Upon blue light irradiation, the autocamtide-2 inhibitory peptide is uncaged, and competitively inhibits CaMKII.
B, ERK monobody binder is a monobody engineered from the fibronectin type III domain to specifically bind the docking site on ERK, preventing ERK interaction with downstream effectors, inhibiting ERK activity.
C, The RapR system achieves temporal regulation of kinase activity via chemically inducible dimerization through the insertion of FKBP, which disrupts the kinase catalytic site. Addition of rapamycin recruits FRB, which binds to FKBP and relieves active site inhibition, enabling kinase activity.
D, Optogenetic regulation of kinase activity by insertion of light-regulated domains into kinases. (i) Kinases can be caged by the inclusion of a dimeric Dronpa within the catalytic domain, where dimerization of Dronpa blocks kinase activity by sterically hindering the active site. Illumination with blue light triggers Dronpa photodissociation and uncaging of the kinase active site, which can be reversed by illumination with violet light. (ii) In LightR, regulation of kinases by light is accomplished through insertion of tandem vivid photoreceptors into the kinase domain backbone. In the dark state, the catalytic site is disrupted, but illumination with blue light induces vivid homodimerization and restoration of the catalytic site.