Fig. 4.
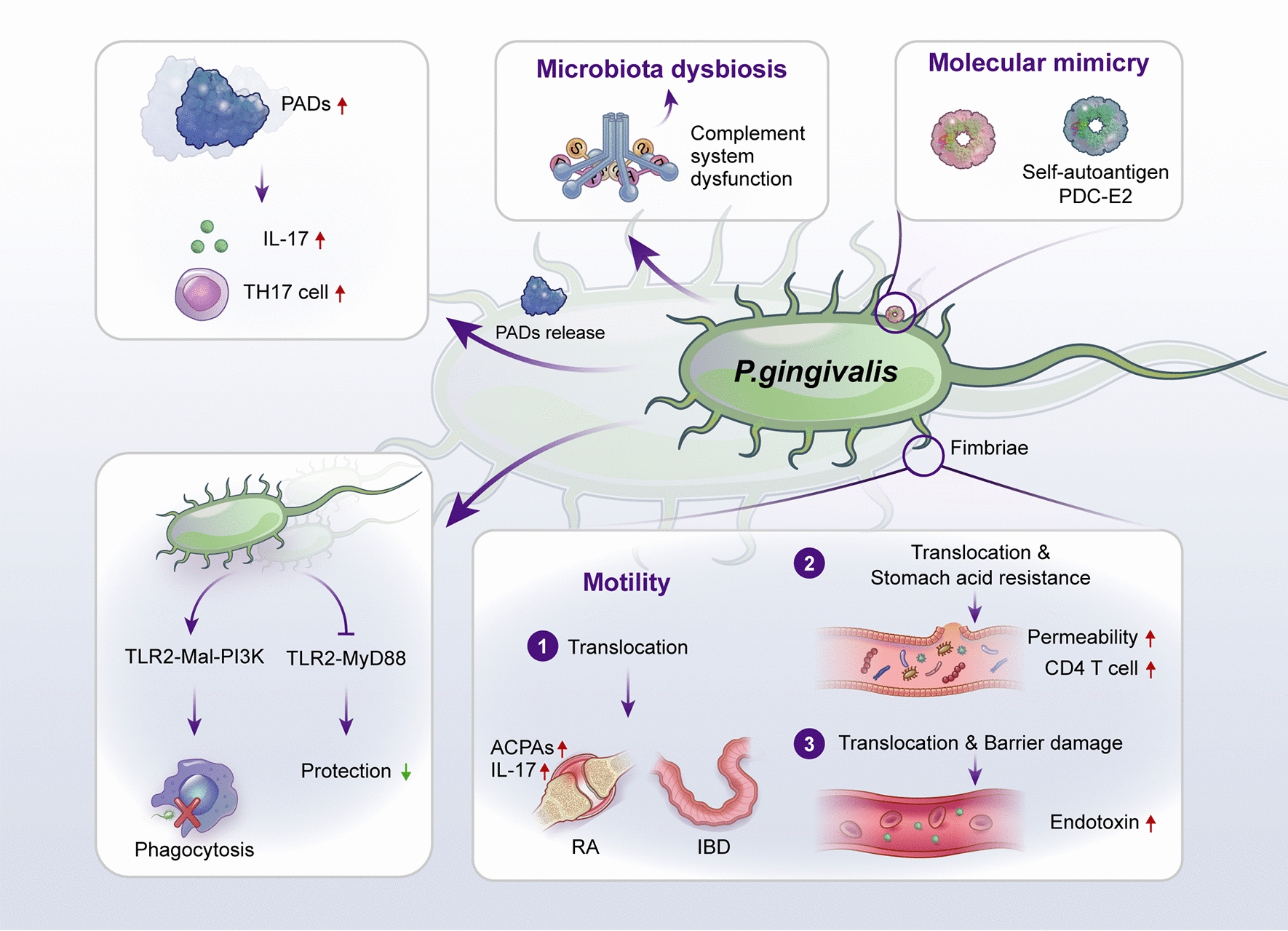
Graphical description of targeting the possible mechanisms of P. gingivalis for triggering autoimmune diseases. P. gingivalis is a Gram-negative anaerobic bacillus, mainly locating in the oral cavity. Bottom right: However, due to barrier damage and the function of withstanding stomach acidity, distant translocation of P. gingivalis into joints and enteral tissue by hematogenous dissemination and enteral route has been found in animal models. After successful colonization and survival, the higher level of endotoxin in blood and IL-17 as well as ACPAs in joints could be found in mice treated with P. gingivalis or mixed oral pathogenic microorganisms containing P. gingivalis. Bottom left: Moreover, P. gingivalis possesses immunosuppressive activities by activating the host-detrimental TLR2-Mal-PI3K pathway to blocked cellular phagocytosis. Also, P. gingivalis inhibited the host-protective TLR2-MyD88, which may further weaken the protective function of the immune system. Top middle: In addition, affecting the normal function of the complement system by P. gingivalis conducive to oral microbiota. Top left: PADs from P. gingivalis could catalyze the citrullination of proteins, which promotes the production of Th17 cells as well as IL-17 and exacerbates UC. Top right: P. gingivalis have similar epitopes as the self-autoantigen PDC-E2, which is the possible mechanism underlying the initiation and progression of primary biliary cirrhosis. ACPAs, anti-citrullinated protein antibodies; PADs, peptidylarginine deiminases; PDC-E2, pyruvate dehydrogenase complex E2
