Figure 1. Synthesis and characterization of BPDs.
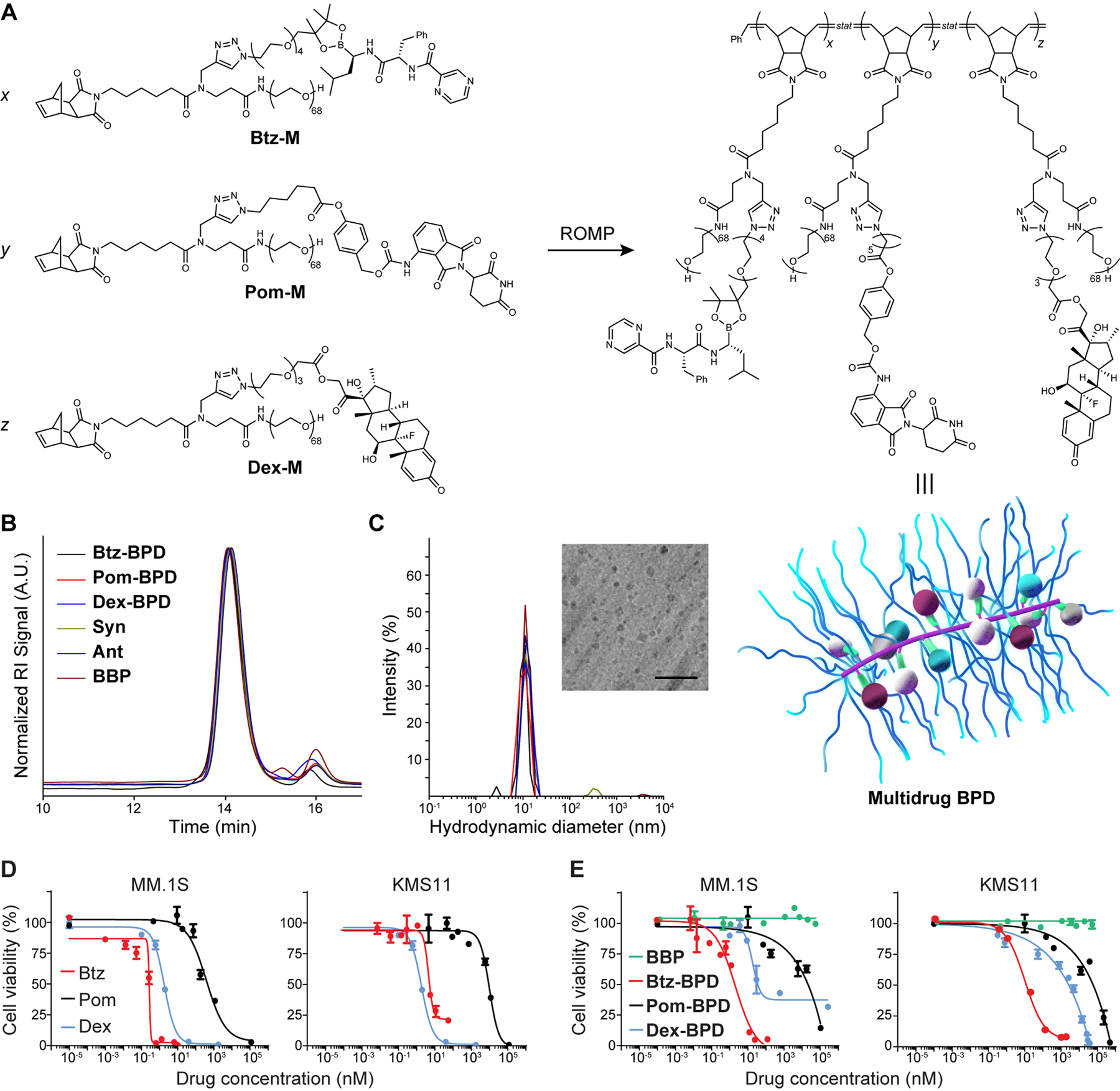
(A) Chemical structures of prodrug macromonomers used in this work. These macromonomers, or mixtures thereof, were subjected to ROMP via exposure to Grubbs 3rd-generation bis-pyridyl complex to produce the corresponding BPDs. Schematic of multidrug BPD is provided (not drawn to scale). Maroon, blue and white spheres = different drugs arranged randomly along the BPD backbone; green = cleavable linkers that activate to release the drugs; purple = BPD backbone; blue strands = poly(ethylene glycol) (PEG) shrouds the drugs and backbone, providing similar physical properties for BPDs regardless of drug identity. (B) Size exclusion chromatography traces of BPDs. The minor peak at 16 min elution time corresponds to residual macromonomers. (C) Hydrodynamic diameters (Dh) of BDPs as determined by dynamic light scattering (DLS). Inset: Cryogenic electronic microscopy image of 3-drug BPD “Syn” (scale bar = 50 nm). (D) Free drugs and (E) 1-drug BPDs were evaluated in MM.1S and KMS11 cell lines (BBP refers to a drug-free bottlebrush polymer). Cell viability (n = 3 biologically independent samples) was evaluated by the CellTiter Glo assay at 48 h after incubation with varying concentrations. Data are presented as mean ± SEM.
