Abstract
Objective
Patients with lung cancer with underlying idiopathic pulmonary fibrosis and usual interstitial pneumonia (UIP) pattern on CT represent a very high-risk group in terms of postoperative UIP acute exacerbations (AEs) and in-hospital mortality. We sought to investigate the outcomes in these patients.
Methods
We carried out a meta-analysis, searching four international databases from 1 January 1947 to 27 April 2022, for studies in any language reporting on the acute postoperative outcomes of patients with lung cancer undergoing surgical resection with underlying UIP (the primary outcome). Random effects meta-analyses (DerSimonian and Laird) were conducted. We analysed the difference in incidence of postoperative AE as well as the difference in long-term overall survival among subpopulations. These were stratified by the extent of surgical resection, with meta-regression testing (uniivariate and multivariate) according to the stage of disease, operative decision making and country of origin. This study was registered with PROSPERO (CRD42022319245).
Results
The overall incidence of AE of UIP postoperatively from 10 studies (2202 patients) was 14.6% (random effects model, 95% CI 9.8 to 20.1, I2=74%). Sublobar resection was significantly associated with a reduced odds of postoperative AE (OR 0.521 (fixed effects model), 95% CI 0.339 to 0.803, p=0.0031, I2=0%). The extent of resection was not significantly associated with overall survival following lung cancer resection in UIP patients (HR for sublobar resection 0.978 (random effects model), 95% CI 0.521 to 1.833, p=0.9351, I2=71%).
Conclusions
With appropriate implementation of perioperative measures such as screening for high-risk cases, appropriate use of steroids, antifibrotics and employing sublobar resection in select cases, the risk of local recurrence versus in-hospital mortality from AEUIP can be balanced and long-term survival can be achieved in a super-selected group of patients. Further investigation in the form of a randomised study is warranted.
Keywords: Thoracic Surgery, Interstitial Fibrosis, Lung Cancer
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Patients with underlying usual interstitial pneumonia who are undergoing surgical resection for early-stage lung cancer represent a high-risk group for perioperative complications.
WHAT THIS STUDY ADDS
In a super-selected, appropriately investigated, and preoptimised group of patients, anatomical lung resection can be safely performed. Judicious use of sublobar resection can be considered in small cancers in patients deemed too high risk for formal lobectomy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
With the advent of worldwide screening incentives for lung cancer, the incidence of this high-risk group of patients is going to rise. Recent randomised data (JCOG0802 and CALGB140503) has highlighted the utility and drawbacks of segmental resection in early-stage lung cancer. Further trial design and subsequent policy change is of utmost importance taking into account the data presented in this study in order to appropriately manage this group of patients.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a specific form of chronic, progressive fibrosing interstitial pneumonia.1 Usual interstitial pneumonia (UIP) is the radiologic pattern seen in patients with IPF. The hallmark of this condition on histopathological criteria is a low magnification appearance of patchy dense fibrosis that results in lung parenchymal remodelling (affecting the subpleural and paraseptal regions most severely), honeycomb appearances and alternates with areas of less affected lung tissue.1 Median survival in this group of patients without treatment is 78 months, with a clear survival difference between usual pattern and non-specific pattern fibrosis.2 Lung biopsy is recommended where there is uncertainty over the diagnosis and when high-resolution CT patterns are not firmly supportive, however, any form of surgical intervention on severely fibrotic lungs carries a risk of postoperative acute exacerbation (AE) and mortality.
In non-small cell lung cancer (NSCLC) patients who require a curative resection, the prevalence of IPF as an associated disease is 3.7% as reported by the Japanese Association for Thoracic Surgery.3 Moreover, the same group conducts annual surveys of the thoracic surgery practice nationally, in 2012, 26 079 patients underwent anatomical lobectomy for lung cancer (73.1% of entire cohort), and AE of UIP postoperatively was the leading cause of postoperative mortality in this group. This was followed by pneumonia, major cardiovascular event, bronchopleural fistulation and respiratory failure.4
AE of UIP in patients with lung cancer is a critical factor in determining postoperative mortality. Groups have reported up to 44% postoperative mortality rates in this group of patients undergoing lung resection for cancer.5 This pattern has been seen following non-surgical interventions also, the incidence of severe radiation pneumonitis and poor prognosis is significantly higher in this group following Stereotactic Ablative Radiotherapy for lung cancer.6 Although much of the work has been done in IPF patients, UIP is the fibrosis associated with IPF and we are going to use this term throughout the study.
In this study, we will seek to address the outcomes in patients undergoing different types of surgical resection with respect to acute in-hospital morbidity and mortality and long-term overall and disease-specific survival. We, thus, performed a meta-analysis of the best available evidence. The current era is a suitable time to assess this, given the rising rates of lung cancer detection given worldwide screening incentives as well as recent randomised data highlighting the utility and drawbacks of segmental resection in early-stage lung cancer, the first of its kind and the largest randomised trial in thoracic surgery (JCOG0802).7
Materials and methods
Protocols and guidance
This study was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).8 The protocol for this review was registered with PROSPERO (CRD42022319245).
Inclusion criteria
We considered studies to be eligible if they enrolled adults (>18 years old) undergoing lung cancer resection in the presence of underlying UIP, whether this be the primary group or indeed a specifically analysed subgroup within the cohort. Studies that included comparative data between extent of lung cancer resection as well as reporting of disease-specific or overall lung cancer survival. We included randomised studies, prospective and retrospective cohort studies, cross-sectional studies, case–control studies and registry data. Studies were considered for inclusion on the basis of the following criteria:
Reporting on surgical lung cancer treatment in adults with underlying UIP.
Reporting on clinical outcome, not protocol or treatment methodology alone (case reports excluded).
Studies containing duplicate data were excluded and manuscripts with the best documented material reporting on patient outcome were used in this review.
Exclusion criteria
We excluded studies if they were case reports; if participants were <18 years of age; if participants did not have underlying UIP and if there was incomplete reporting on outcome data including conference abstracts.
Outcomes
The primary outcome was the incidence of AE of UIP in the postoperative period following lung cancer resection. An AE was defined as per the standard criteria proposed by Collard et al 9; sudden acceleration of disease or acute injury superimposed on already diseased lung in the 30-day postoperative period after having excluded infection, left ventricular failure, pulmonary embolism and other identifiable causes of acute lung injury.10 Secondary outcome included overall survival.
Search strategy
We devised a systematic search algorithm (online supplemental figure 1) in order to interrogate the literature and determine the optimal management for patients with underlying UIP in need of lung resection. We searched MEDLINE, EMBASE, LILACS and Cochrane Library databases using the OVID interface. We reviewed articles from 2000 onwards and restricted this to full-text papers only. We did not impose any language restrictions. We retrieved over 3000 articles (online supplemental figure 2, PRISMA flow chart) and screened 100 full-text articles, from which we determined 49 full-text articles to be eligible for inclusion in this study. We deemed these to represent the best evidence concerning the role of lung resection with underlying UIP.
bmjresp-2022-001529supp001.pdf (47.6KB, pdf)
bmjresp-2022-001529supp002.pdf (411.4KB, pdf)
Study selection
After removal of duplicates, two independent reviewers screened all titles and abstracts. Screening results from both reviewers were compared, and where conflicts arose, disputes were settled by discussion with the lead authors. Full-text publications were retrieved for studies deemed to be suitable.
Data collection process
A standardised, prepiloted form was used to extract data from the included studies for the assessment of study quality and for evidence synthesis. Extracted information included study setting; study population and participant demographics and baseline characteristics; details of the intervention and type of surgery; study methodology; outcomes and times of measurement; information for assessment of the risk of bias.
Studies not meeting these criteria, or positively meeting the exclusion criteria were not included in the analysis; reason for exclusion was recorded. Discussion between the reviewing authors was used to resolve conflicts in paper exclusion; where necessary, final decision was determined by consensus. As per the PRISMA guidance, the selection process was fully documented.8 11 The characteristics of studies included in the meta-analysis were recorded (online supplemental table 2).
bmjresp-2022-001529supp004.pdf (193.1KB, pdf)
Risk of bias assessment
Two researchers (AJP and ESB) independently assessed the quality of all included studies by using the Cochrane Collaboration risk of bias tool.12
Data synthesis and analysis
We performed statistical analyses the Meta package in R (V.4.0.3; R Project for Statistical Computing). Analyses for all outcomes were conducted on an intention to treat basis. We used ORs and HRs and their associated 95% CIs to assess outcomes and considered a p value less than 0.05 to be statistically significant. For those studies where there was a difference in reference category, we manipulated the data by taking the inverse of the HRs and CIs to standardise the reference category for analysis purposes (marked in online supplemental table 2). We assessed heterogeneity using Q statistics and the I2 test.13 If significant heterogeneity was not present (I2<50%), we used fixed effects models to pool outcomes; we used random effects models when significant heterogeneity was present (I2≥50%). The possibility of small study effects was assessed qualitatively by visual estimate of the funnel plot and quantitatively by calculation of the Egger’s test.14
Subgroup analyses
Where there was significant heterogeneity (I2≥50%) present, we conducted univariate and multivariate meta-regression in order to determine the impact of various factors on the true effect sizes seen. These included country of study origin, stage of NSCLC and the decision making behind the extent of resection. This also informed us whether there were significant interactions to perform subgroup analyses.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing of results. The results will be disseminated to a wide audience, including members of the public, patients, health professionals and experts in the specialty through social media and networks.
Results
See PRISMA diagram (online supplemental figure 2) for a description of included studies in the meta-analysis.
Primary outcome: postoperative AE of UIP
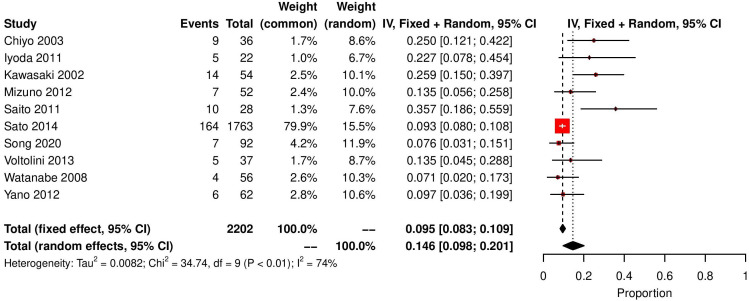
Ten studies reported AE rates of UIP postoperatively, 231 out of 2202 patients experienced an AE in hospital. Proportional meta-analysis determined the rate of AE post lung cancer resection as 14.6% (random effects model, 95% CI 9.8 to 20.1, I2=74%) (figure 1).
Figure 1.
Forest plot demonstrating incidence of AE of UIP in pantiens with lung cancer. AE, acute exacerbation; UIP, usual interstitial pneumonia.
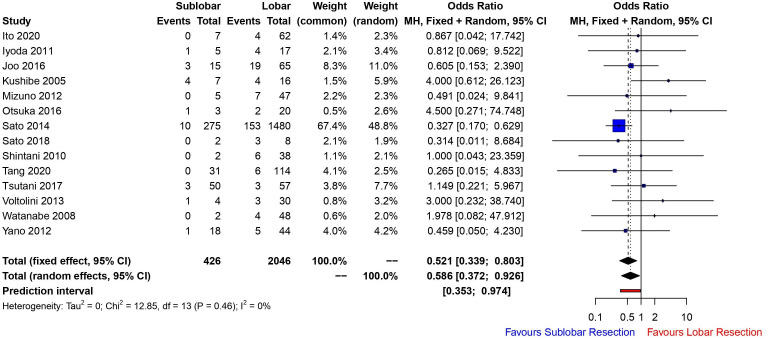
Fourteen studies compared lobar versus sublobar resection with regard to postoperative AE of UIP. Sublobar resection was significantly associated with a reduced odds of postoperative AE (OR 0.521 (fixed effects model), 95% CI 0.339 to 0.803, p=0.0031, I2=0%) (figure 2). Funnel plot analysis showed no asymmetry, additionally the Egger’s test (p=0.2454) detected no significant small study effects. We were unable to meta-analyse the in-hospital mortality rate in this group by the extent of lung resection owing to poor reporting of these data and heterogeneity among reporting time frames.
Figure 2.
Forest plot demonstrating incidence of AE of UIP in patients with lung cancer stratified according to extent of lung resection (sublobar vs lobar resection). AE, usual interstitial pneumonia; UIP, usual interstitial pneumonia.
Two studies compared segmental versus wedge resection with regard to postoperative AE of UIP. Segmental resection was associated with an increased odds of postoperative AE (OR 1.874 (fixed effects model), 95% CI 0.727 to 4.834, p=0.194, I2=0%) (figure 3), although not significant. This is representative of the lower sample number and fewer studies which stratified outcomes within the sublobar resection category.
Figure 3.
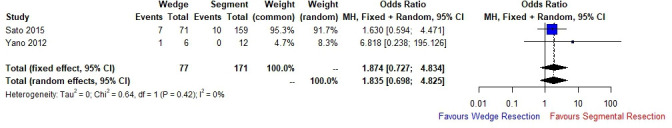
Forest plot demonstrating incidence of AE of UIP in patients with lung cancer stratified according to extent of lung resection (segmentectomy vs wedge resection). AE, usual interstitial pneumonia; UIP, usual interstitial pneumonia.
Secondary outcome: overall survival
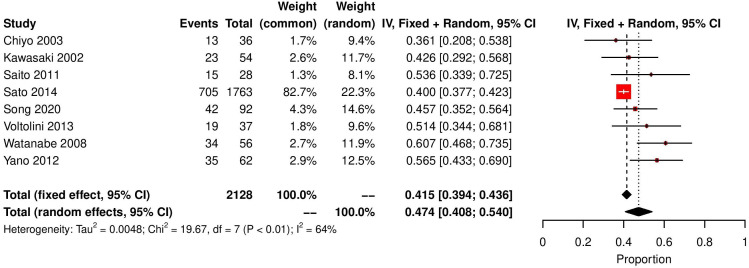
Eight studies reported 5-year overall survival in this unique group of patients, 886 out of 2128 patients survived to 5 years post lung cancer resection. Proportional meta-analysis determined the overall survival to be 47.4% at 5 years (random effects model, 95% CI 40.84 to 54, I2=64.4%) (figure 4). As for the rates of postoperative AE (figure 1), the levels of heterogeneity were significant which is not surprising. Factors such as stage of cancer, choice of lung resection, preoperative and postoperative treatments for cancer and UIP are accounted for below.
Figure 4.
Forest plot demonstrating overall survival of patients with lung cancer with underlying UIP following curative resection. UIP, usual interstitial pneumonia.
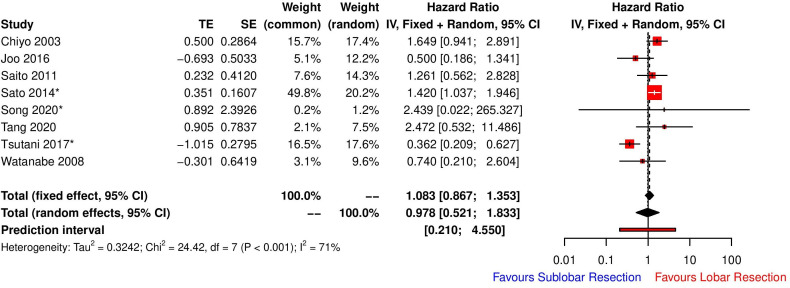
The extent of resection was not significantly associated with overall survival following lung cancer resection in UIP patients (HR for sublobar resection 0.978 (random effects model), 95% CI 0.521 to 1.833, p=0.9351, I2=71%) (figure 5). The high heterogeneity among studies was explored with a view to determine the clinical basis of the heterogeneity. Factors such as country, stage of NSCLC and the decision making behind the extent of resection were explored in a univariate meta-regression model. Neither country (Japan/China/South Korea) nor decision making (random/surgeon or centre preference vs proper risk stratification determining extent of resection) behind surgical strategy accounted for the high heterogeneity (no change to I2 test for unexplained variability and R2 (amount of heterogeneity accounted for) = 0%). However, we found that 50.06% of the difference in true effect sizes can be explained by the stage of NSCLC of the patient cohort in each study, although not significant (R2=50.06%, test of residual heterogeneity, p=0.06, test of moderators, p=0.186). When combining both stage of NSCLC and decision making in a multivariate meta-regression model, 94.07% of the difference in true effect sizes can be explained (test of moderators, p=0.0042) with no significant residual heterogeneity (p=0.363).
Figure 5.
Forest plot demonstrating overall survival of patients with lung cancer with underlying UIP following curative resection stratified according to extent of lung resection. *For those studies where there was a difference in reference category, we manipulated the data by taking the inverse of the HRs and CIs to standardise the reference category for analysis purposes. CI, confidence interval; HR, hazard ratio; UIP, usual interstitial pneumonia.
Egger’s linear regression test of funnel plot asymmetry yielded a p value of 0.7236, implying no publication bias, however, n number was <10, therefore, this may lack statistical power. Only one study reported disease-free survival, hence we were unable to meta-analyse owing to a lack of data. Sublobar resection has been shown, however, to be significantly associated with a shorter disease-free interval compared with lobar resection across all cancer stages (HR 4.74, 95% CI 1.12 to 20.06, p=0.03).15
Discussion
UIP and lung cancer
The incidence of lung cancer in UIP is well described; with up to 50% of patients with UIP developing some form concurrent pulmonary malignancy.16 The incidence was significantly higher than in the age-matched general population without UIP (48.2% vs 9.1%, p<0.001).17 This is not altogether surprising given that there are underlying molecular and genetic aberrations common to both disease processes.18 Yoo et al demonstrated that cumulative year on year risk for malignancy in UIP increased at a near exponential rate; 1 year for lung cancer was 1.1%, whereas at 10 years this was 31.1%.19 Male gender, current smoker at diagnosis and a rapid annual decline of more than 10% in forced vital capacity (FVC) were associated with increased risk of lung cancer.19
Postoperative outcomes in lung cancer and underlying UIP
Online supplemental table 2 illustrates the major studies20 21 35 23 24 25 26 which have explored the impact of underlying UIP for patients who undergo major lung resection for NSCLC. Despite having a variable prevalence, some groups reporting up to 13%,19 UIP is consistently recognised as a significant independent negative prognostic factor in terms of postoperative pulmonary complications, in-hospital mortality and overall survival (online supplemental table 2). The impact of UIP on disease-free interval must be interpreted cautiously, some groups have reported no difference27 whereas others have demonstrated a significantly worse disease free interval with underlying UIP.28 This must be stratified according to the type of surgical resection as lesser resections are often employed in UIP patients to protect against disease AE postoperatively. As we shall explore later, lesser resections do associate with higher rates of locoregional recurrence. Sato et al 5 29 have presented the largest series of concomitant NSCLC and UIP (n=1763) to date and showed that death due to cancer was the main cause of mortality (50.2%), followed by respiratory failure (26.8%).
Our analysis has demonstrated that the rate of AE post lung cancer resection as 14.6% (random effects model, 95% CI 9.8 to 20.1, I2=74%) (figure 1), which is a significant proportion particularly when AE is a significant predictor of in-hospital mortality.5 16 29–34 Eight studies reported 5-year overall survival in this unique group of patients, 886 out of 2128 patients survived to 5 years post lung cancer resection with random effects testing yielding a 5-year survival rate of 47.4%. This again must be interpreted with caution as proper stratification according to stage of disease, degree of fibrosis, type of fibrosis and resection extent must be accounted for.
In the pretreatment decision-making phase, careful attention should be paid to the type of interstitial lung disease (ILD) present on the radiology, for example, non-specific interstitial pneumonia (NSIP) carries a much better prognosis and this should be radiologically classified making the distinction between fibrotic type NSIP and true UIP. The prognosis of NSIP is significantly better than that of UIP; 5-year survival of 43% among patients with UIP, compared with 90% in NSIP and this is similarly reflected in those patients undergoing major lung resections for cancer.2 3
Although we did not perform a robust risk factor analysis for AE in these patients (ie, duration of surgery, oxygenation levels, vent settings, FVC, severity of UIP, Karnofsky status and comorbidities of patients), this has already been well described by Hao et al 35 who demonstrated a series of factors that confer higher risk in these patients. Of note, UIP pattern on CT conferred an OR of 1.52 for postoperative AE (95% CI 1.06 to 2.17, p=0.021)35; not all studies confirmed clear clinical or biopsy proven diagnoses of UIP, some studies reported a blend of ILD.5 29 These factors are critical to the prognostic algorithm in this group of patients.
The role of limited resection
Limited surgical resection in lung cancer has been increasingly adopted in recent years, for early stage solid and subsolid lesions, particularly in light of the findings from the recent JCOG0802 randomised controlled trial7 which assessed the 5-year outcomes following segmentectomy (sublobar) (n=552) or lobectomy (n=554) for stage Ia NSCLC less than or equal to 2 cm. Overall survival was 94.3% and 91.1%, respectively (p=0.0082), yet there was no significant difference in disease-free survival; 88% and 87.9%, respectively. The proportion of locoregional recurrence in the segmentectomy arm was significantly higher than the lobectomy arm, 10.5% vs 5.4% (p=0.0018), respectively. In this particular field, there is limited scope to delineate between segmentectomy and lobectomy hence the discussion will also compare sublobar (wedge resection and segmentectomy combined) to lobar resection, which is a reflection of the paucity of large-scale robust data.
The guidance suggests that there is a role for surgery in the setting of high-risk patients with UIP36 although with a significantly lower 5-year survival rate (54.2%–61.6% for stage I NSCLC) and late death from respiratory failure.30 31 37 Retrospective data suggest that limited sublobar resection (wedge or segmentectomy) is better tolerated in this group given that there is a reduced incidence of AE, shorter postoperative hospital stay and reduced rate of air leak.16 29 34 Across 14 studies analysing 2472 patients, sublobar resection was significantly associated with a reduced odds of postoperative AE (OR 0.521 (fixed effects model), 95% CI 0.339 to 0.803, p=0.0031, I2=0%) (figure 2). This could be due to a number of different factors, reduced operative time, less handling of the lung and less lung tissue resected. We must bear in mind, however, that without level I evidence, this cannot be formally accepted as experienced centres performing segmentectomy do report that this operation is associated with more tissue handling and longer operating times particularly if performed minimally invasively. Moreover, the air leak rate following segmentectomy can be higher than a lobectomy, as the fibrotic lungs of patients with clinically significant IPF are typically very difficult to staple across the parenchyma for a sublobar resection. Two studies3 29 did stratify postoperative AE of UIP according to segmental and wedge resection; segmental resection was associated with higher odds of postoperative AE (OR 1.874) when compared with wedge resection, although this was not significant.
Wedge resection also associated with lower mortality from respiratory failure compared with lobectomy but conferred a poorer long-term prognosis (5-year survival of 33.2% compared with 68.4% with lobectomy).29 Small cohort retrospective data have shown significantly higher disease free survival following lobar resection compared with sublobar resection for early-stage lung cancer in patients with underlying UIP.15 However, we must bear in the mind recent robust randomised data from JCOG0802,7 which demonstrated a 5-year relapse-free survival rate of 88.0% (95% CI 85.0% to 90.4%) for segmentectomy and 87.9% (95% CI 84.8% to 90.3%) for lobectomy (HR 0.998; 95% CI 0.753 to 1.323; p=0.9889) for early-stage lung cancer, despite higher local recurrence in the segmentectomy group. Moreover, data from CALGB-140503 has shown comparable 5-year disease-free survival between sublobar and lobar resection groups (63.6% vs 64.1%, p=0.018 for non-inferiority), with 59% of the sublobar resection cases being wedge resections. The use of wedge resection therefore could be of fundamental importance to patients with concurrent UIP both in terms of short and long-term cancer-specific survival.
Our analysis did not demonstrate a significantly reduced long-term survival with sublobar resection (HR 0.978 (random effects model), 95% CI 0.521 to 1.833, p=0.9351, I2=71%) (figure 5). However, further meta-regression has suggested that in super-selected cases with early-stage small tumours that have gone through a proper risk stratification and counselling process may have comparable long-term survival to patients undergoing lobectomy. As there was no survival difference, it is highly likely that most sublobar resections were wedge resections and survival cannot be reliably controlled for the stage of disease and extent of resection given the paucity of reported data.
A delicate balance must be struck in order to ensure these patients have a long disease-free interval without local recurrence at the cost of significant postoperative mortality from IPF AE. Patient selection is key and opting for limited resection in patients with FVC<80% and low DLco are factors that can guide management. Furthermore intraoperative measures such as reducing the time on single-lung ventilation, avoiding fluid overload and hyperoxic time are also measures to be considered.33 Groups have described minimally invasive operative techniques as a means of mitigating the risk of postoperative AE.38 The role of limited resection in this setting and its association with a lower rate of AE must be correlated with the poorer oncological outcomes and is indeed the focus of an ongoing randomised study.39
Conclusions
Postoperative AEUIP in patients with lung cancer confers a significant risk of in-hospital morbidity and mortality. This can be offset with judicious use of sublobar resection, and appropriate implementation of perioperative measures. In a super-selected group of patients, long-term survival can be achieved and this would also form the crux of a recruitment cohort that should be subject to a randomised controlled trial, which would seek to definitely address the issues we have highlighted in this manuscript.
Strength and limitations
This meta-analysis has several methodological strengths. We followed the recommendations of the Cochrane Collaboration and PRISMA statement, including a priori protocol. It is the first meta-analysis in this particular area which will importantly help to address key issues and guide management when dealing with this high-risk cohort. There is no level I randomised evidence in this space, addressing outcomes or indeed utility of prophylaxis measures to offset postoperative morbidity and mortality. The study encountered a paucity of trials reporting on uniform mortality rates and disease-free interval stratified according to extent of resection. Data for this secondary outcome might have been collected differently than data for the primary outcome in the studies. The vast majority of patients come from a single series,5 29 which may influence the overall effect size of the analysis.
bmjresp-2022-001529supp003.pdf (39.4KB, pdf)
Footnotes
Contributors: AJP and ESB designed the experimental plan. AJP carried out processing and data analysis. AJP and ESB interpreted the results and constructed and designed the manuscript. GIW, SW, VR, HF, MK and BN provided constructive feedback to the design and layout of the manuscript. AJP Is acting as guarantor of this work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data are publicly available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–68. 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 2. Riha RL, Duhig EE, Clarke BE, et al. Survival of patients with biopsy-proven usual interstitial pneumonia and nonspecific interstitial pneumonia. Eur Respir J 2002;19:1114–8. 10.1183/09031936.02.00244002 [DOI] [PubMed] [Google Scholar]
- 3. Yano M, Sasaki H, Moriyama S, et al. Post-Operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg 2012;14:146–50. 10.1093/icvts/ivr029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masuda M, Kuwano H, Okumura M, et al. Thoracic and cardiovascular surgery in japan during 2012: annual report by the japanese association for thoracic surgery. Gen Thorac Cardiovasc Surg 2014;62:734–64. 10.1007/s11748-014-0464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604–11. 10.1016/j.jtcvs.2013.09.050 [DOI] [PubMed] [Google Scholar]
- 6. Finazzi T, Ronden M, Nossent E, et al. MA02.02 toxicity of lung SABR in patients with coexisting interstitial lung disease. Journal of Thoracic Oncology 2019;14:S252. 10.1016/j.jtho.2019.08.503 [DOI] [Google Scholar]
- 7. Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607–17. 10.1016/S0140-6736(21)02333-3 [DOI] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636–43. 10.1164/rccm.200703-463PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juarez MM, Chan AL, Norris AG, et al. Acute exacerbation of idiopathic pulmonary fibrosis-a review of current and novel pharmacotherapies. J Thorac Dis 2015;7:499–519. 10.3978/j.issn.2072-1439.2015.01.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chapter 7: considering bias and conflicts of interest among the included studies. n.d. Available: https://training.cochrane.org/handbook/current/chapter-07
- 13. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joo S, Kim DK, Sim HJ, et al. Clinical results of sublobar resection versus lobectomy or more extensive resection for lung cancer patients with idiopathic pulmonary fibrosis. J Thorac Dis 2016;8:977–84. 10.21037/jtd.2016.03.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iyoda A, Azuma Y, Sakamoto S, et al. Surgical treatment for patients with idiopathic pulmonary fibrosis and lung cancer: postoperative acute exacerbation of idiopathic pulmonary fibrosis and outcomes. Surg Today 2022;52:736–44. 10.1007/s00595-021-02343-0 [DOI] [PubMed] [Google Scholar]
- 17. Matsushita H, Tanaka S, Saiki Y, et al. Lung cancer associated with usual interstitial pneumonia. Pathol Int 1995;45:925–32. 10.1111/j.1440-1827.1995.tb03417.x [DOI] [PubMed] [Google Scholar]
- 18. Tzouvelekis A, Gomatou G, Bouros E, et al. Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest 2019;156:383–91. 10.1016/j.chest.2019.04.114 [DOI] [PubMed] [Google Scholar]
- 19. Yoo H, Jeong BH, Chung MJ, et al. Risk factors and clinical characteristics of lung cancer in idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med 2019;19:149. 10.1186/s12890-019-0905-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiyo M, Sekine Y, Iwata T, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg 2003;126:1141–6. 10.1016/s0022-5223(03)00791-8 [DOI] [PubMed] [Google Scholar]
- 21. Ito H, Nakayama H, Yokose T, et al. A prophylaxis study of acute exacerbation of interstitial pneumonia after lung cancer surgery. Jpn J Clin Oncol 2020;50:198–205. 10.1093/jjco/hyz164 [DOI] [PubMed] [Google Scholar]
- 22. Iyoda A, Jiang S-X, Amano H, et al. Prediction of postoperative exacerbation of interstitial pneumonia in patients with lung cancer and interstitial lung disease. Exp Ther Med 2011;2:1073–6. 10.3892/etm.2011.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kushibe K, Kimura M, Takahama M, et al. Lung resections for lung cancer with idiopathic pulmonary fibrosis. Kyobu Geka 2005;58:26–30. [PubMed] [Google Scholar]
- 24. Mizuno Y, Iwata H, Shirahashi K, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2012;41:e161–5. 10.1093/ejcts/ezs147 [DOI] [PubMed] [Google Scholar]
- 25. Otsuka H, Sugino K, Hata Y, et al. Clinical features and outcomes of patients with lung cancer as well as combined pulmonary fibrosis and emphysema. Mol Clin Oncol 2016;5:273–8. 10.3892/mco.2016.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang H, Ren Y, She Y, et al. Is operation safe for lung cancer patients with interstitial lung disease on computed tomography? Ther Adv Respir Dis 2020;14:1753466620971137. 10.1177/1753466620971137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawasaki H, Nagai K, Yoshida J, et al. Postoperative morbidity, mortality, and survival in lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol 2002;81:33–7. 10.1002/jso.10145 [DOI] [PubMed] [Google Scholar]
- 28. Song MJ, Kim DJ, Paik HC, et al. Impact of idiopathic pulmonary fibrosis on recurrence after surgical treatment for stage I-III non-small cell lung cancer. PLoS One 2020;15:e0235126. 10.1371/journal.pone.0235126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sato T, Watanabe A, Kondo H, et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015;149:64–9. 10.1016/j.jtcvs.2014.08.086 [DOI] [PubMed] [Google Scholar]
- 30. Watanabe A, Higami T, Ohori S, et al. Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J Thorac Cardiovasc Surg 2008;136:1357–63, 10.1016/j.jtcvs.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 31. Saito Y, Kawai Y, Takahashi N, et al. Survival after surgery for pathologic stage Ia non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann Thorac Surg 2011;92:1812–7. 10.1016/j.athoracsur.2011.06.055 [DOI] [PubMed] [Google Scholar]
- 32. Shintani Y, Ohta M, Iwasaki T, et al. Predictive factors for postoperative acute exacerbation of interstitial pneumonia combined with lung cancer. Gen Thorac Cardiovasc Surg 2010;58:182–5. 10.1007/s11748-009-0569-z [DOI] [PubMed] [Google Scholar]
- 33. Voltolini L, Bongiolatti S, Luzzi L, et al. Impact of interstitial lung disease on short-term and long-term survival of patients undergoing surgery for non-small-cell lung cancer: analysis of risk factors. Eur J Cardiothorac Surg 2013;43:e17–23. 10.1093/ejcts/ezs560 [DOI] [PubMed] [Google Scholar]
- 34. Tsutani Y, Mimura T, Kai Y, et al. Outcomes after lobar versus sublobar resection for clinical stage I non-small cell lung cancer in patients with interstitial lung disease. J Thorac Cardiovasc Surg 2017;154:1089–96. 10.1016/j.jtcvs.2017.03.116 [DOI] [PubMed] [Google Scholar]
- 35. Hao X, Hao J, Chen C, et al. Risk factors for acute exacerbation of interstitial lung disease following lung cancer resection: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2022;34:744–52. 10.1093/icvts/ivab350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Homma S, Bando M, Azuma A, et al. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig 2018;56:268–91. 10.1016/j.resinv.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 37. Sato S, Shimizu Y, Goto T, et al. Survival after repeated surgery for lung cancer with idiopathic pulmonary fibrosis: a retrospective study. BMC Pulm Med 2018;18:134. 10.1186/s12890-018-0703-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tane S, Ando Y, Matsumoto G, et al. Basal segment deep wedge resection for lung cancer with pulmonary fibrosis. Gen Thorac Cardiovasc Surg 2022;70:413–5. 10.1007/s11748-021-01764-5 [DOI] [PubMed] [Google Scholar]
- 39. Tanaka K, Tsutani Y, Wakabayashi M, et al. Sublobar resection versus lobectomy for patients with resectable stage I non-small cell lung cancer with idiopathic pulmonary fibrosis: a phase III study evaluating survival (JCOG1708, surprise). Jpn J Clin Oncol 2020;50:1076–9. 10.1093/jjco/hyaa092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001529supp001.pdf (47.6KB, pdf)
bmjresp-2022-001529supp002.pdf (411.4KB, pdf)
bmjresp-2022-001529supp004.pdf (193.1KB, pdf)
bmjresp-2022-001529supp003.pdf (39.4KB, pdf)
Data Availability Statement
All data are publicly available.