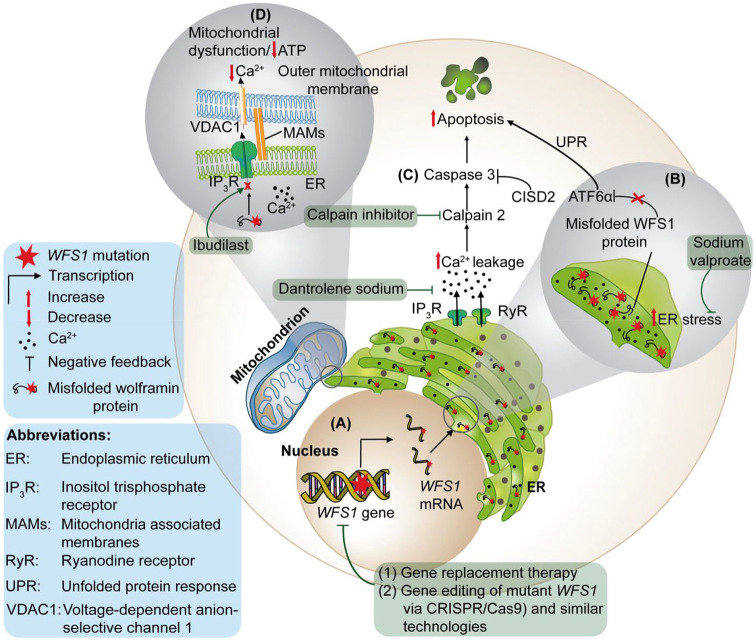
Figure 2.
Pathophysiological mechanisms implicated in Wolfram Syndrome. (a) A mutation in the WFS1 gene can lead to loss of function (with degradation of the mutant mRNA transcript) or result in an aberrant wolframin protein with a dominant-negative effect or a gain-of-function. In this diagrammatic representation, the mutant mRNA transcript generates a misfolded protein that is mislocalised, leading to increased levels of ER stress. (b) Sodium valproate is a drug targeting ER stress that has been shown to increase the expression of p21, which is crucial for cell survival under conditions of heightened ER stress. In healthy cells, wolframin negatively regulates ATF6α, which is a key protein regulating the UPR. Deficiency of wolframin is thought to increase ATF6α signaling, resulting in upregulation of the UPR and increased apoptosis. (c) Wolframin plays a central role in maintaining ER homeostasis and in controlling intracellular Ca2+ flux. Loss of wolframin increases cytosolic Ca2+ and this ultimately triggers apoptosis. The disruption of Ca2+ flux leads to hyperactivation of calpains, which are calcium-dependent cysteine proteases, in particular calpain 2. As a result, there is increased caspase-3 cleavage. CISD2 is a negative regulator of calpain 2 that limits the cleavage of caspase-3. ER Ca2+ stabilizers can normalize ER homeostasis by targeting ER calcium transporters such as the ryanodine receptors. Dantrolene sodium and calpain inhibitors normalize cytosolic Ca2+ and calpain activity by inhibiting ER Ca2+ efflux via the ryanodine receptors, which helps to suppress apoptosis. (d) Wolframin activates IP3R-mediated Ca2+ release and it promotes ER-mitochondria Ca2+ transfer by binding to the NCS1 to form a complex with IP3R. Loss of wolframin triggers NCS1 degradation and IP3R dysfunction leading to decreased mitochondrial Ca2+ uptake, causing mitochondrial dysfunction and a reduction in ATP production. The physical interaction of mitochondria with the ER is established through MAMs. MAMs serve as close contact sites facilitating Ca2+ transfer via IP3R on the ER, which interacts with VDAC1 on the outer mitochondrial membrane. Wolframin deficiency results in disorganization of the MAMs and a reduction in mitochondrial Ca2+ uptake. Ibudilast restores the resting cytosolic Ca2+ levels through its interaction with NCS1.
ER, endoplasmic reticulum; IP3R, inositol triphosphate receptor; MAMs, mitochondria-associated membranes; NCS1, neuronal Ca2+ sensor-1; UPR, unfolded protein response; VDAC1, voltage-dependent anion-selective channel protein 1.