Figure 3.
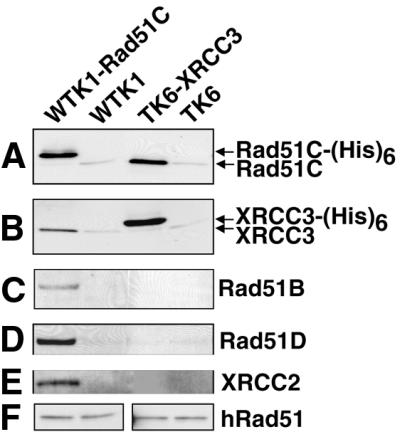
Interactions of XRCC3 and Rad51C, and of Rad51C with Rad51B, Rad51D and XRCC2 in human cells. Following Ni2+ pull down of recombinant Rad51C-(His)6 and XRCC3-(His)6 from untreated cells, western blot analysis was used to determine which of the Rad51 paralogs interacted constitutively. Rad51C-(His)6 is directly pulled down in the first lane, and XRCC3-(His)6 is directly pulled down in the third lane. WTK1 and TK6 (second and fourth lanes) are negative controls. (A) Western blot using antibody against Rad51C (from P. Sung). (B) Western blot using antibody against XRCC3 (P. Sung). (C–E) Western blots using antibodies against Rad51B (J. Albala), Rad51D (Novus) and XRCC2 (P. Sung), respectively, showing that these proteins are associated with Rad51C-(His)6, but not with XRCC3-(His)6. (F) Western blots using antibody against Rad51 (Calbiochem). A small fraction of native Rad51C, XRCC3 and Rad51 were non-specifically bound to the Ni2+ beads (A, B and F).
