Figure 5. Filament formation stabilizes the C-terminus and allosteric site.
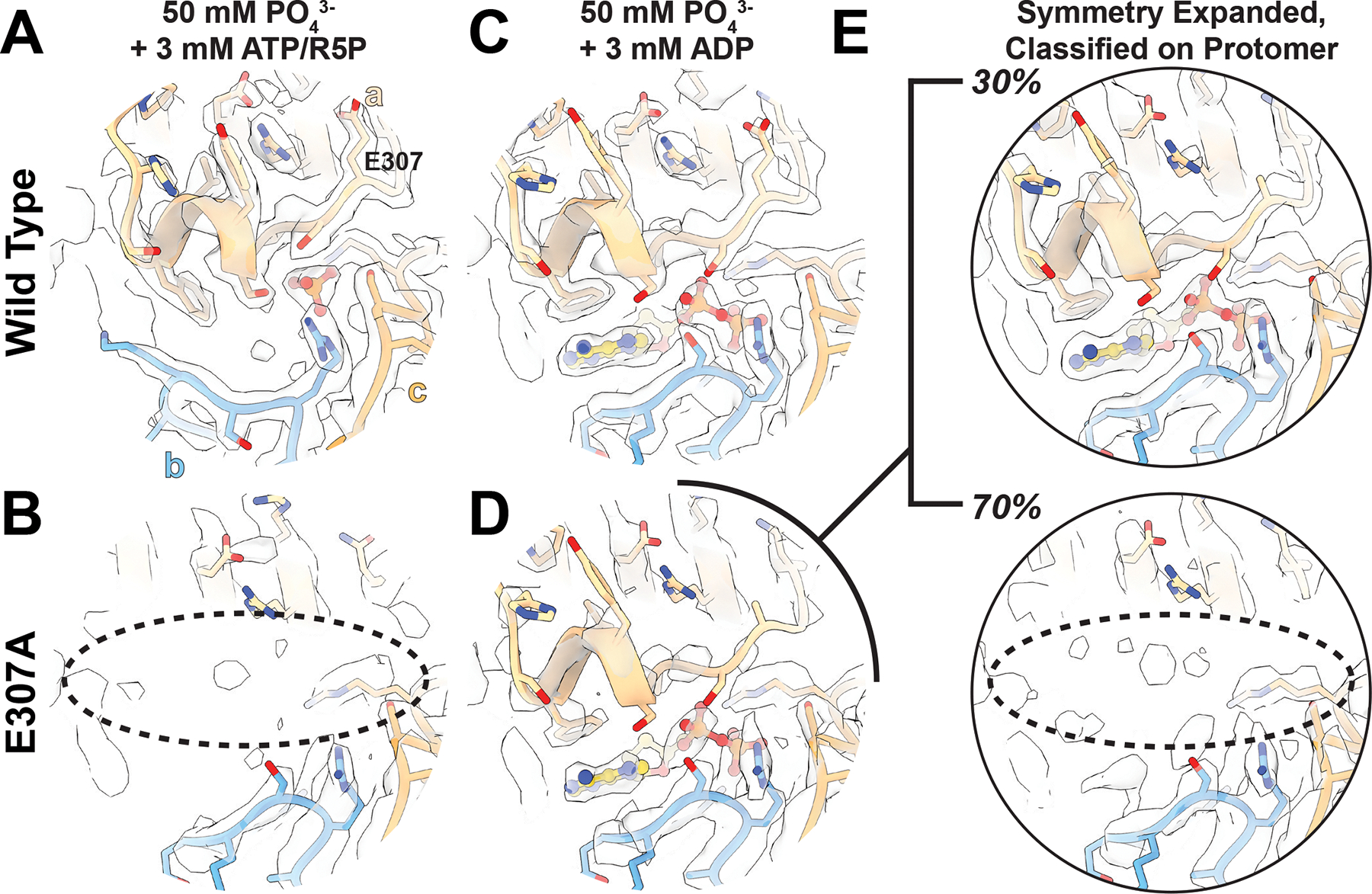
A. The allosteric site and C-terminus of PRPS1 in the presence of phosphate, ATP, and ribose-5-phosphate. B. The allosteric site and C-terminus of PRPS1-E307A. C. The allosteric site and C-terminus of PRPS1 in the presence of phosphate and ADP. D. The allosteric site and C-terminus of PRPS1-E307A in the presence of phosphate and ADP. E. The PRPS1-E307A dataset was symmetry expanded and classified without alignment on a masked protomer, and two volumes were locally refined. Top. Map and model of the PRPS1-E307A showing the presence of the C-terminus and ADP bound in the allosteric site. Bottom. Map and model of the PRPS1-E307A lacking ADP in the allosteric site and a bound C-terminus.
