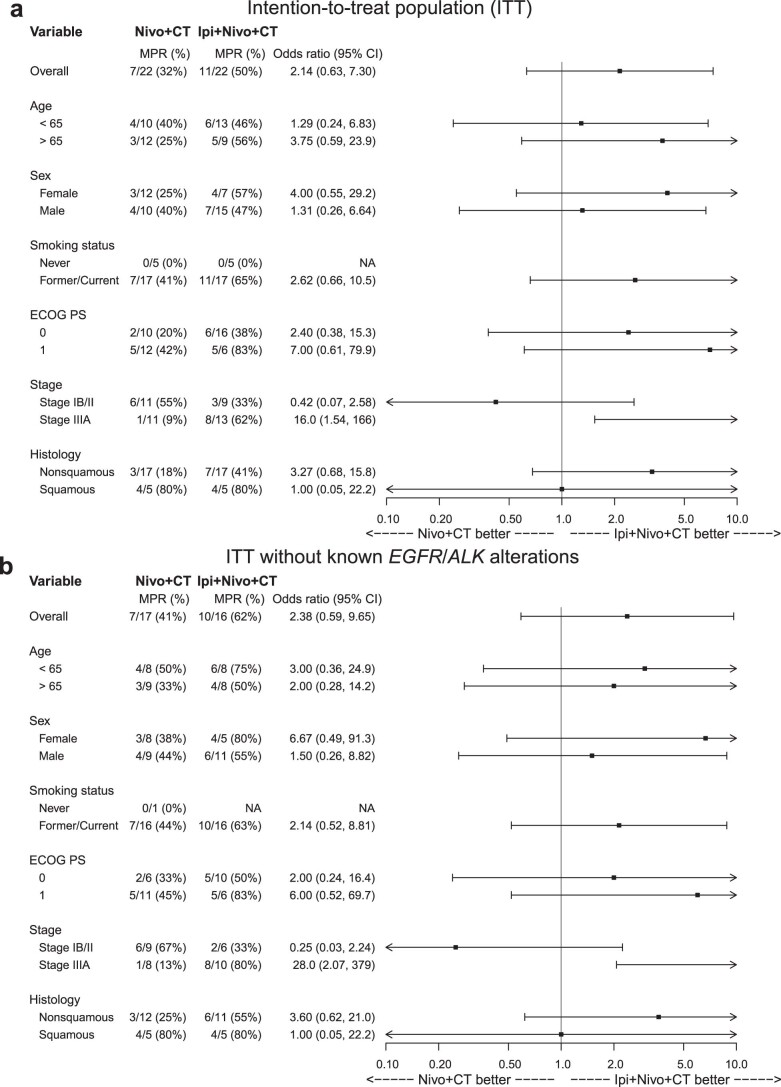
Extended Data Fig. 2. Impact of clinicopathological characteristics on efficacy of neoadjuvant Nivo+CT and Ipi+Nivo+CT.
a, Forest plot of the odds ratio (95% confidence interval) to explore the association between treatment arm and MPR for each subgroup (Nivo+CT, n = 22; Ipi+Nivo+CT, n = 22). b, Forest plot of the odds ratio (95% confidence interval) to explore the association between treatment arm (Nivo+CT, n = 17; Ipi+Nivo+CT, n = 16) and MPR for each subgroup in patients without known tumor EGFR/ALK alterations. In both panels, the vertical reference lines at 1 indicate no difference between two treatment arms. The point estimates of odds ratios are represented by solid squares. The whiskers are the two lines that extend to the lower and upper bounds of the 95% confidence intervals. The lower and upper limits of the 95% confidence intervals are clipped at 0.1 and 10.0 to arrows. The odds ratios and 95% confidence interval are from univariate logistic regression. MPR, major pathologic response; Nivo, nivolumab; Ipi, ipilimumab; CT, chemotherapy; Never, never smoker; Former/Current, former/current smoker; ECOG, Eastern Cooperative Oncology Group; PS, performance status; Squamous, squamous cell carcinoma; Nonsquamous includes adenocarcinoma, carcinoma with neuroendocrine features, NOS NSCLC, sarcomatoid carcinoma, and large cell carcinoma; NA, not available.