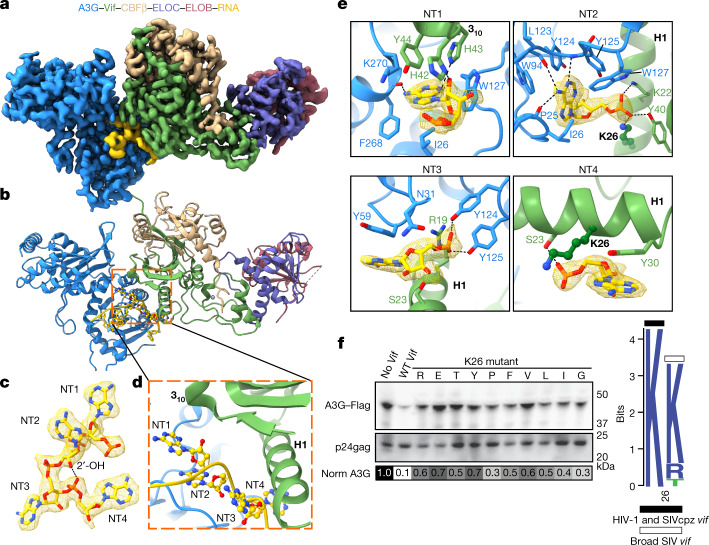
Fig. 1. Structure of the VCBC ligase substrate receptor in complex with human A3G and RNA.
a, Cryo-EM map for the A3G–RNA–VCBC monomer. b, Corresponding view of the refined coordinate model of the A3G–VCBC complex, highlighting the four-nucleotide core motif (ball-and-stick) between Vif and A3G. Here and throughout, the same colour coding for A3G, Vif, CBFβ, ELOB, ELOC and RNA is used as indicated. c, Composite density map for NT1–4 of RNA, with a hydrogen bond indicated between ribose 2′-OH on NT2 and phosphate on NT4. d, Ribbon diagram showing NT1–4 of RNA bridging helix 1 (H1) and 310 helix turn of Vif with A3G. e, Close-up of protein–RNA interactions between Vif and A3G for each core nucleotide of RNA. f, Functional assessment of amino acid substitutions of residue K26 in HIV-1 Vif. Left, amino acid mutants at Vif residue K26 were assessed for their ability to prevent packaging of A3G into virions; top, virion incorporation of A3G; bottom, amount of virus (p24gag) in the corresponding virion preparation. Below is a greyscale heatmap of relative A3G incorporation normalized to p24gag based on two replicate transfections (with the exception of K26Y), with the amount of A3G in the ‘No Vif’ control set to 1.0 (darkest shading). Controls were run on the same gel as the samples. For Source data, see Supplementary Fig. 1. Right, logo plot of amino acids found in the consensus of all HIV-1 clades, as well as SIVcpz (black bar) and all other SIV strains with equal distribution of each SIV (white bar). WT, wild type.