Abstract
Background:
Little is known about the effect of exercise modality during a dietary weight loss program on muscle size and quality, as measured by computed tomography (CT). Even less is known about how CT-derived changes in muscle track with changes in volumetric bone mineral density (vBMD) and bone strength.
Methods:
Older adults (66±5 years, 64% women) were randomized to 18-months of diet-induced weight loss (WL), WL with aerobic training (WL+AT), or WL with resistance training (WL+RT). CT-derived muscle area, radio-attenuation and intermuscular fat percentage at the trunk and mid-thigh were determined at baseline (n=55) and 18-month follow-up (n=22–34), and changes were adjusted for sex, baseline value, and weight lost. Lumbar spine and hip vBMD and finite element-derived bone strength were also measured.
Results:
After adjustment for the weight lost, muscle area losses at the trunk were −7.82 cm2 [−12.30, −3.35] for WL, −7.72 cm2 [−11.36, −4.07] for WL+AT, and −5.14 cm2 [−8.65, −1.63] for WL+RT (p<0.001 for group differences). At the mid-thigh, decreases were −6.20 cm2 [−10.39, −2.02] for WL, −7.84 cm2 [−11.19, −4.48]) for WL+AT, and −0.60 cm2 [−4.14, 2.94] for WL+RT; this difference between WL+AT and WL+RT was significant in post-hoc testing (p=0.01). Change in trunk muscle radio-attenuation was positively associated with change in lumbar bone strength (r=0.41, p=0.04).
Conclusions:
WL+RT better preserved muscle area and improved muscle quality more consistently than WL+AT or WL alone. More research is needed to characterize the associations between muscle and bone quality in older adults undertaking weight loss interventions.
Keywords: randomized controlled clinical trial, finite element bone strength, sarcopenia, bone density, exercise modality
1. INTRODUCTION
By 2040 there will be about 82.3 million Americans aged 65 and older,1 and at least a third of them are projected to live with obesity.2 Primary lifestyle interventions to manage obesity include caloric restriction and exercise, which are known to induce weight loss,3 improve mobility,4 and reduce cardiovascular disease risk.5 Concomitant muscle and bone loss, however, along with the potential to exacerbate age-related risk of sarcopenia and osteoporosis, has stalled widespread clinical weight loss recommendation for this demographic.6–8
Evidence from randomized controlled weight loss trials conducted in older adults suggests that concurrent resistance training may modestly prevent loss of muscle and bone mass,9–11 usually measured by dual energy x-ray absorptiometry (DXA). However, DXA alone does not capture all properties of muscle and bone that contribute to their strength.12 Computed tomography (CT) is increasingly used to complement DXA imaging in clinical studies because it provides high-resolution 3D measures of morphological muscle quality (“fatty infiltration” or inter- and intramuscular fat deposition) and bone density and strength. For instance, increased intermuscular and intramuscular fat deposition (indicative of poorer muscle quality), measured in CT, has been associated with mobility limitations,13 lower muscle strength independent of muscle mass,14 increased fracture risk,15 increased likelihood of incident falls,16 and mortality.17 Furthermore, lifestyle interventions, such as nutritional supplementation and physical activity, have been shown to influence positively the muscle composition of older adults with mobility limitation.18 Despite evidence supporting its use as a biomarker, studies examining the effect of exercise type on CT-derived muscle quality measures in older adults undergoing diet-induced weight loss is currently lacking.
In addition to muscle quality metrics, CT also provides information about bone quality. CT-derived bone metrics like volumetric bone mineral density (vBMD) and bone strength estimated through finite element (FE) modeling have been successfully used for fracture-risk assessment.19 Previous studies suggest that muscle mass is associated with bone health in older adults.20,21 In addition to muscle mass, higher calf muscle density (indirect measure of inter- and intra-muscular fat) has been associated with better bone health cross-sectionally16 and paraspinal muscle fat fraction has been associated with lower lumbar vBMD,22 but it has not been examined directly whether changes in muscle quality are associated with changes in bone strength, nor whether these local bone-muscle health associations track longitudinally or apply to other regions such as the hip. If muscle quality were an additional contributor to bone strength in older adults, then interventions that improve or preserve muscle quality might also be preferentially indicated for bone health. Indeed, data from an epidemiological study in older men shows that appendicular lean mass is associated with bone CT parameters independently of muscle strength and power,23 highlighting the influence of other muscle properties. Muscle and bone are not only coupled via mechanotransduction, which stretches collagen fibers and periosteum at the muscle-bone interface; their mechanical and endocrine interaction also involves pleiotropic genes and secretory factors, such as IGF-1, myostatin, osteocalcin, irisin, osteopontin, sclerostin, and others.24–27
Leveraging the platform provided by the Cooperative Lifestyle Intervention Program-II (CLIP-II; NCT01547182) randomized controlled trial (RCT), for which treatment effects on DXA-derived body composition and CT-derived vBMD and bone strength outcomes have been published,11,28–30 the primary purpose of this exploratory analysis is to characterize the effect of a dietary weight loss program alone, with aerobic training, or with resistance training on CT-derived trunk and mid-thigh cross-sectional skeletal muscle area (CSA), muscle radio-attenuation, intermuscular fat deposition, and skeletal muscle index (SMICT: CSA/[patient height]2). The secondary purpose is to examine how changes in CT-derived muscle area and muscle radio-attenuation associate to changes in CT-derived bone metrics (vBMD and finite element-derived bone strength).
2. METHODS
2.1. Study Sample
Details of the CLIP-II design and its methods are published,31 as well as the primary outcome paper that includes the full trial inclusion/exclusion criteria and CONSORT diagram.32 Briefly, CLIP-II was an 18-month RCT that included 249 older adults (66.8±4.7 years) with obesity (33.8±3.6 kg/m2), cardiovascular disease (CVD; 26.1%) and/or metabolic syndrome (84.3%), and self-reported mobility disability (self-reported difficulty with walking ¼ mile), with dual primary outcomes of mobility (400-m walk time) and muscle strength (knee extensor strength). Participants were randomized to diet-induced weight loss only (WL), weight loss plus aerobic training (WL+AT) or weight loss plus resistance training (WL+RT) in a community-based setting, with the goal of eliciting 7–10% body mass loss. The caloric deficit was ~330 kcals/day during the intensive phase (months 1–6), followed by a transition phase (months 7–12), and maintenance phase (months 13–18). The caloric deficit aims of the transition phase depended on the progress of the individual, but during this phase the focus moved gradually towards weight maintenance for the last six months, with emphasis on portion control and improving the quality of food choices. WL+AT consisted of walking for 45-minutes/day with an intensity of 12–14 on the Borg Rating of Perceived Exertion Scale four days/week, and WL+RT involved upper and lower body machine-based exercise performed for 45-minutes/day with 3 sets of 10–12 repetitions at 75% of 1 repetition maximum four days/week with progressive overload. As reported in the main outcome paper, WL plus exercise groups improved 400 meter walk time (~17 second reduction) and knee extensor strength normalized by body mass (~15% improvement) as compared to WL alone, with no difference between AT or RT groups.32
This present analysis focuses on a subset of these participants that belong to the last two recruitment phases (waves 7 and 8) who consented to receive a thigh and a lumbar CT scan at baseline (n=55) and 18-month follow-up (n=34). Helical CT scans of the abdominal trunk and both thighs were acquired on a 64-slice scanner (LightSpeed VCT, General Electric Medical Systems, Milwaukee, WI) at 120 kVp and 250 mA, with a slice thickness of 2.5mm for the trunk and 0.625mm for the thighs. Sample sizes for the CT-derived muscle and bone outcomes are reported in eFigure 1. The larger CLIP-II study population and the CT subset were very similar with regard to age (66.8 ± 4.7 vs 65.8 ± 4.3 years), sex (71.1% vs 63.6% female), race (32.1% vs. 27% Black), and body mass index (BMI; 33.8 ± 3.6 vs 34.0 ± 3.5 kg/m2).32 All participants provided written informed consent prior to enrollment (IRB00018631).
2.2. CT-acquired Muscle Measures
A single CT slice was selected from the abdominal trunk at the middle of the third lumbar vertebral level (L3; half of the vertebral body height)33 and another single CT slice from the mid-thigh. The mid-thigh was defined as equidistant between the lesser trochanter and the intercondylar fossa,34 using the right femur as measurement reference on the CT scouts for placement consistency. The selected CT slices were analyzed by a blinded, trained investigator using Mimics (v.23; Materialise, Leuven, Belgium). The abdominal trunk and the mid-thigh are both body regions widely used in sarcopenia and exercise research and are clinically relevant in the assessment of bone health.35,36 Mid-thigh data is reported as the average of both legs, and trunk data is reported bilaterally.
For each body region, skeletal muscle tissue was segmented by thresholding within the range of −29 to 150 Hounsfield Units (HU) and intermuscular fat between −190 to −30 HU.37 Thresholding operations were followed by manual tracing to exclude visceral content, skin, and bone. Adipose tissue depots located between muscle fiber bundles and within the deep fascial boundary of whole muscle surfaces were segmented as intermuscular fat areas.38 The intermuscular fat percentage was calculated by dividing the intermuscular adipose tissue area by the sum of the total muscle area and the intermuscular adipose tissue area. Figure 1 shows example segmentations of female participants with the same BMI but contrasting muscle areas and intermuscular fat percentages. Muscle radio-attenuation, which indirectly quantifies lipid droplets within muscle fibers, was measured as the average HU from the muscle area.
Figure 1.
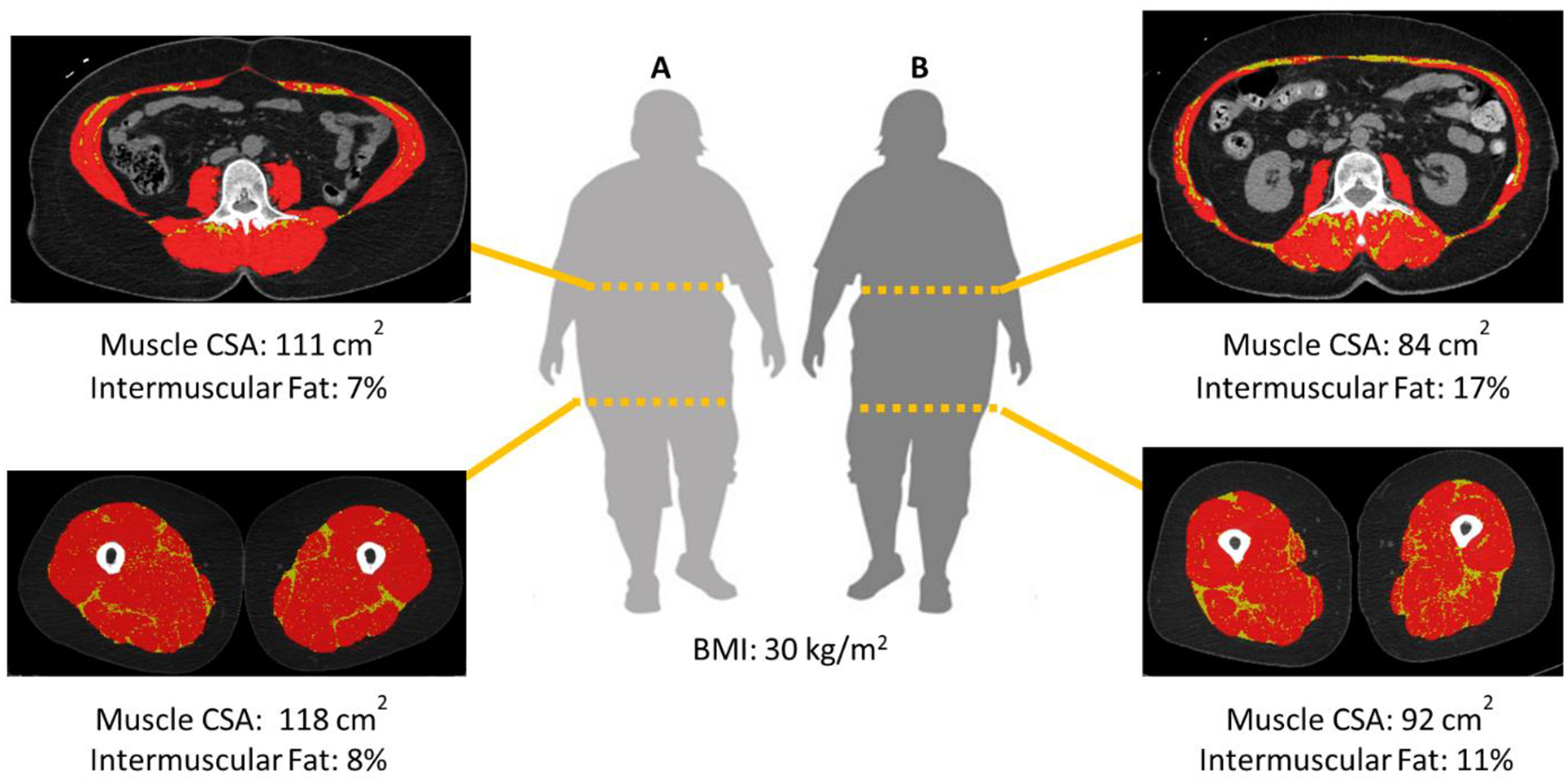
Examples of trunk and mid-thigh CT segmentations. The red area highlights the skeletal muscle cross-sectional area (CSA; −29 to 150 HU) and the yellow area highlights the intermuscular fat (−190 to −30 HU).
A random sample of 10% (n=6) of the baseline study cohort was analyzed thrice by the same investigator, and the coefficient of variation was <2% for muscle measures and <6% for fat measures. Inter-reader intra-class correlation coefficients for CT-derived muscle measurements collected by our group range from 0.94 to 0.99, indicating excellent reliability.39
2.3. CT Skeletal Muscle Index of Sarcopenia
The SMICT (cm2/m2) was obtained by normalizing trunk muscle area by each participant’s height squared.40,41 SMICT cut-offs for sarcopenia used were 38.5 cm2/m2 for women and 52.4 cm2/m2 for men.41
2.4. CT-acquired Bone Measures
The methods for the measurement of vBMD and of the finite element modeling-derived bone strength have been previously described for this cohort.11,29,30 To explore muscle-bone associations, the following bone outcomes were used: mean integral vBMD of the L1–L4 vertebrae, mean integral vBMD of the total hip (left and right average), compressive strength averaged for the L1–L4 vertebrae, and hip strength in a configuration simulating impact from a sideways fall30,42 (referred to here as “fall strength”; left and right average). Bone strength is a function of geometry, cortical thickness, and vBMD30 and the higher the strength in a particular configuration, the higher the resistance to fracture in that configuration.
2.5. Statistical Analysis
Baseline characteristics were summarized as means and standard deviations (mean ± SD) for continuous variables or counts and percentages [n (%)] for discrete variables. Data was analyzed using SAS (v.9.4. SAS Institute Inc., Cary, NC) and all statistical testing was two-sided and based on a 5% probability level. The 18-month changes were calculated by subtracting the baseline values from follow-up values; therefore, a negative mean change denotes a decrease from baseline to follow-up. As assumptions of independence, homogeneity of variance, and normality of experimental errors were confirmed, intervention effects on CT-derived muscle measures were estimated using a general linear model fit with treatment group, sex, and wave, adjusted for baseline value of each muscle outcome (Model 1). To generate Model 2, Model 1 was further adjusted for weight change over 18 months. Pairwise Tukey tests were used when the generalized linear model F-test indicated the presence of linear contrast among the means. Associations of the CT-derived muscle measures with vBMD and bone strength were estimated by partial Pearson correlation coefficients (r) adjusted for sex, recruitment wave, and baseline value of the bone variable.
3. RESULTS
3.1. Participant Characteristics
Baseline age, sex, race, body mass, and BMI for the 55 subgroup participants are reported in Table 1. The 34 participants with 18-month follow-up CT data were demographically similar to the baseline sample and the characteristics remained balanced among randomization groups (eTable 1). In accordance with the goals of the trial, all three treatment groups lost significant weight. Total body mass changes adjusted for baseline body mass were mean [95% CI]: −6.14 kg [−11.76, −0.52] for WL alone; −11.74 kg [−16.24, −7.23] for WL+AT; and −9.05 kg [−14.01, −4.08] for WL+RT; no difference between groups was found (p=0.13). Expressed as a percentage of baseline body weight, the difference between WL and WL+AT was −6.2% [−15.4, 2.9], between WL and WL+RT was −4.2% [−13.5, 5.1], and between WL+AT and WL+RT the difference was 2.0% [−6.4, 10.5]; no difference between groups was found (p=0.35).
Table 1.
Baseline descriptive characteristics and attendance data by treatment group and overall. WL=Weight loss alone; WL+AT=WL plus aerobic training; WL+RT=WL plus resistance training; kg=kilogram, m=meter. Continuous data are presented as mean (SD) and categorical variables are presented as n (%).
| Baseline Variable | WL (n=17) | WL + AT (n=19) | WL + RT (n=19) | All (n=55) |
|---|---|---|---|---|
| Age, years | 66.8 (3.9) | 65.8 (5.1) | 65.0 (3.8) | 65.8 (4.3) |
| Female | 11 (65) | 12 (63) | 12 (63) | 35 (64) |
| Race/ethnicity | ||||
| Black | 6 (35) | 6 (32) | 3 (16) | 15 (27) |
| Hispanic | 0 | 0 | 1 (5) | 1 (2) |
| White | 11 (65) | 13 (68) | 14 (74) | 38 (69) |
| Other/Mixed/Missing | 0 | 0 | 1 (5) | 1 (2) |
| Total body mass, kg | 97.8 (18.8) | 93.6 (16.1) | 97.0 (16.5) | 96.1 (16.9) |
| Body Mass Index, kg/m2 | 34.4 (3.6) | 33.6 (3.5) | 34.1 (3.7) | 34.0 (3.5) |
| Intervention Session Attendance, % | 65.9 (35.6) | 75.7 (24.7) | 76.8 (15.8) | 73.1 (26.2) |
3.2. Intervention Effects on CT Muscle Measures
Intervention effects on muscle measures are shown in Table 2. At the trunk, WL+RT lost mean [95% CI]: −4.97 cm2 [−9.30, −0.63] of muscle CSA, while WL+AT lost −8.14 cm2 [−12.64, −3.63] and WL lost −5.24 cm2 [−10.54, 0.04]). At the mid-thigh WL+RT experienced a loss of −0.57 cm2 [−5.10, 3.96] while WL+AT lost −8.85 cm2 [−13.11, −4.60] and WL lost −3.94 cm2 [−9.12, 1.25]); this difference between WL+AT and WL+RT was significant in post-hoc testing (p=0.01). Following adjustment for weight lost, muscle area losses remained attenuated in WL+RT (−5.14 [−8.65, −1.63] at trunk; −0.60 [−4.14, 2.94] at mid-thigh), while both WL and WL+AT experienced similar muscle area losses at the trunk (WL: −7.82 cm2 [−12.30, −3.35]; WL+AT: −7.72 cm2 [−11.36, −4.07]), and mid-thigh (WL: −6.20 cm2 [−10.39, −2.02]; WL+AT: −7.84 cm2 [−11.19, −4.48]). Both WL+RT and WL+AT tended to increase trunk muscle radio-attenuation, while WL alone tended to decrease it (p=0.01 for between-group comparisons in the weight loss-adjusted model), although post hoc testing did not reveal pairwise differences (eTable 2). When expressed as a percentage of the muscle area and adjusted for weight lost, the reductions in intermuscular fat percentage of WL+RT were comparable to those of WL+AT at the trunk (WL+RT: −1.24% [−2.34, −0.14]; WL+AT: −1.31% [−2.36, −0.25]), and were higher than those experienced by both WL alone and WL+AT at the mid-thigh (WL+RT: −2.59% [−3.71, −1.47]; WL: −2.07% [−3.44, −0.70]; WL+AT: −2.07% [−3.13, −1.01]; p<0.01 between group, no pairwise differences in post hoc testing). The ratio of muscle area to fat area lost at the trunk is 3.2:1 for WL, 2.7:1 for WL+AT, and 2.0:1 for WL+RT. The ratio of muscle to fat area losses at the mid-thigh are 1.6:1 for WL, 1.9:1 for WL+AT, and 0.2:1 for WL+RT.
Table 2.
Unadjusted baseline and adjusted 18-month intervention effects on computed tomography muscle metrics. WL= Weight loss alone; WL+AT= WL plus aerobic training; WL+RT= WL plus resistance training; kg= kilogram; m= meter; cm= centimeter; HU= Hounsfield unit; %= percentage. Aggregate baseline data presented as raw mean (SD). 18-month values presented as model adjusted means (95% confidence interval).
| Outcome Variable | Baseline (n=55) | WL (n=9) | WL + AT (n=13) | WL + RT (n=12) | p-value |
|---|---|---|---|---|---|
| Trunk | |||||
| Muscle Area, cm2 | 145.0 (39.5) | ||||
| Δ Model 1a | −5.24 (−10.54, 0.04) | −8.14 (−12.64, −3.63) | −4.97 (−9.30, −0.63) | <0.001 | |
| Δ Model 2b | −7.82 (−12.30, −3.35) | −7.72 (−11.36, −4.07) | −5.14 (−8.65, −1.63) | <0.001 | |
| Muscle Attenuation, HU | 30.5 (7.0) | ||||
| Δ Model 1 | −1.21 (−3.86, 1.43) | 1.44 (−0.63, 3.51) | 0.64 (−1.57, 2.85) | 0.31 | |
| Δ Model 2 | −0.57 (−2.84, 1.69) | 0.68 (−1.12, 2.49) | 0.34 (−1.53, 2.22) | 0.01 | |
| Intermuscular Fat Area, cm2 | 17.44 (12.1) | ||||
| Δ Model 1 | −1.59 (−4.13, 0.95) | −3.61 (−5.61, −1.60) | −2.81 (−4.95, −0.67) | 0.23 | |
| Δ Model 2 | −2.43 (−4.43, −0.42) | −2.89 (−4.48, −1.30) | −2.55 (−4.22, −0.89) | <0.001 | |
| Intermuscular Fat, % | 10.9 (5.7) | ||||
| Δ Model 1 | −0.41 (−1.95, 1.12) | −1.69 (−2.90, −0.49) | −1.36 (−2.65, −0.08) | 0.38 | |
| Δ Model 2 | −0.85 (−2.19, 0.48) | −1.31 (−2.36, −0.25) | −1.24 (−2.34, −0.14) | 0.02 | |
| SMICT, cm2/m2 | 50.3 (11.5) | ||||
| Δ Model 1 | −2.54 (−4.43, −0.65) | −3.26 (−4.84, −1.68) | −2.41 (−3.97, −0.86) | <0.001 | |
| Δ Model 2 | −3.32 (−4.76, −1.87) | −2.80 (−3.99, −1.60) | −2.26 (−3.42, −1.10) | <0.001 | |
| Mid-thigh | |||||
| Muscle Area, cm2 | 124.6 (31.2) | ||||
| Δ Model 1 | −3.94 (−9.12, 1.25) | −8.85 (−13.11, −4.60) | −0.57 (−5.10, 3.96) | 0.006 | |
| Δ Model 2 | −6.20 (−10.39, −2.02) | −7.84 (−11.19, −4.48) | −0.60 (−4.14, 2.94) | <0.001 | |
| Muscle Attenuation, HU | 49.9 (3.4) | ||||
| Δ Model 1 | −0.66 (−2.13, 0.81) | 0.39 (−0.74, 1.52) | 0.77 (−0.44, 1.99) | 0.40 | |
| Δ Model 2 | −0.45 (−1.90, 1.01) | 0.19 (−0.92, 1.31) | 0.70 (−0.48, 1.88) | 0.25 | |
| Intermuscular Fat Area, cm2 | 16.0 (7.8) | ||||
| Δ Model 1 | −3.05 (−5.58, −0.51) | −4.83 (−6.85, −2.81) | −3.85 (−6.01, −1.69) | <0.001 | |
| Δ Model 2 | −3.85 (−5.92, −1.77) | −4.17 (−5.83, −2.52) | −3.69 (−5.43, −1.95) | <0.001 | |
| Intermuscular Fat, % | 11.3 (3.9) | ||||
| Δ Model 1 | −1.51 (−3.27, 0.25) | −2.59 (−3.95, −1.23) | −2.73 (−4.18, −1.27) | 0.05 | |
| Δ Model 2 | −2.07 (−3.44, −0.70) | −2.07 (−3.13, −1.01) | −2.59 (−3.71, −1.47) | <0.001 | |
Model 1 treatment effects were estimated using a generalized linear model fit with treatment group, sex, and wave, adjusted for baseline value of each outcome. Pairwise Tukey comparisons reveal no difference among groups, except between mid-thigh muscle area changes in WL+RT and WL+AT (p=0.02).
Model 2 adjusts for Model 1 and weight change. Pairwise Tukey comparisons reveal no difference among groups, except between mid-thigh muscle area changes in WL+RT and WL+AT (p=0.01).
3.3. Intervention Effects on CT Skeletal Muscle Index of Sarcopenia
At baseline, average SMICT was 45.6 ± 6.9 cm2/m2 for women and 60.2 ± 7.0 cm2/m2 for men. Prior to adjustment for weight change, WL+AT seemed to decline the most SMICT units, while WL+RT seemed to decline the least before and after adjustment for weight change (both p<0.001 between groups, Table 2); but we did not find pairwise differences in any model (p=0.45–0.82, eTable 2). Prevalence of sarcopenia as measured by SMICT was 15% (n=8) at baseline, and only half of these participants follow-up data. From the four participants with follow-up data, only one in the WL+RT group approached the threshold for becoming non-sarcopenic. Among the 30 participants that did not classify as sarcopenic at baseline, one in WL alone, two in WL+RT, and three in WL+AT had sarcopenia at follow-up. Therefore, prevalence of sarcopenia at follow-up was 29% (n=10).
3.4. Associations between Muscle and Bone Quality Measures
Intervention effects on the bone quality outcomes have been published.11 For participants with follow-up data (n=29), baseline vBMD was 123.77 ± 35.54 mg/cm3 and lumbar bone strength was 3.52 ± 1.08 kN. Unadjusted 18-month changes were −3.64 (−6.40, −0.87) mg/cm3 (p=0.12) and −0.17 (−0.33, −0.01) kN (p=0.04), respectively. Baseline total hip vBMD and hip fall strength for participants with follow-up data was 299.15 ± 30.77 mg/cm3 (n=25) and 2.02 ± 0.35 kN (n=22), respectively. Unadjusted 18-month changes were −23.83 (−29.06, −18.60) mg/cm3 (p<0.01) and −0.03 (−0.04, −0.01) kN (p<0.01), respectively. After adjustment for sex, recruitment wave, and baseline value of the bone variable, changes in trunk muscle radio-attenuation were positively correlated to changes in bone strength of the lumbar spine (r=0.41, p=0.04, Table 3). eTable 3 presents cross-sectional associations between muscle and bone health metrics at baseline and at follow-up. At baseline and follow-up, mid-thigh muscle area was significantly associated to hip bone strength in a fall configuration (r=0.67, p<0.01 at baseline and r=0.53, p<0.01 at follow-up), although after adjusting for sex the association at baseline weakened and at follow-up it became non-significant.
Table 3.
Correlations between 18-month changes in computed tomography-derived muscle and bone measures during weight loss
| Δ Lumbar vBMD, mg/cm3 (n=29) | p-Value | Δ Lumbar Compressive Strength, kN (n=29) | p-Value | |
|---|---|---|---|---|
| Trunk | ||||
| Δ Muscle Area, cm2 | 0.24 (−0.17, 0.57) | 0.25 | −0.11 (−0.47, 0.29) | 0.61 |
| Δ Muscle Attenuation, HU | 0.33 (−0.09, 0.65) | 0.12 | 0.41 (0.01, 0.70) | 0.04 |
| Δ Hip vBMD, mg/cm3 (n=25) | p-Value | Δ Hip Fall Strength, kN (n=22) | p-Value | |
| Mid-Thigh | ||||
| Δ Muscle Area, cm2 | 0.31 (−0.13, 0.65) | 0.17 | −0.11 (−0.54, 0.36) | 0.66 |
| Δ Muscle Attenuation, HU | 0.31 (−0.13, 0.65) | 0.17 | 0.37 (−0.10, 0.71) | 0.12 |
Note. Δ= 18-month change; mg= milligram; cm= centimeter; kN= kilo newton; HU= Hounsfield unit; %= percentage; m= meter. Data presented as Pearson correlation estimates (95% confidence interval) adjusted for sex, wave, and baseline value of the bone variable.
4. DISCUSSION
The primary objective of this investigation was to begin to estimate the effect of exercise modality during a community-based intentional weight loss program on multiple regional CT-derived measures of muscle in older adults with obesity. Here we report that WL+RT may be more effective than either WL alone or WL+AT at consistently mitigating muscle size and quality losses. Specifically, RT was able to preserve about 2% (~3 cm2) more trunk muscle area than AT, and about 6% (~7 cm2) more at the mid-thigh. Although Tukey comparisons revealed no significant pairwise differences between groups at the trunk, our finding that adding RT to WL prevents muscle loss in older adults undergoing a weight loss program is in agreement with previously reported whole-body DXA outcomes for the larger CLIP-II cohort28 and from a recent 6-month RCT.43 Further, when matched for total weight lost, there was little difference between WL alone and WL+AT in muscle area loss at either region (expressed as a percentage from baseline, trunk: −5.4% vs. −5.3%; mid-thigh: −5.0% vs. −6.3%). Tied to the latter, six participants who were not sarcopenic at baseline were classified as sarcopenic at follow-up, as measured by SMICT. It is important to note, however, that the three participants from the WL only and the WL+RT groups that became sarcopenic had baseline SMICT just two units above the threshold (i.e., approximately 40.5 cm2/m2), as opposed to the three participants in the WL+AT group, which experienced sharp declines of 4–8 cm2/m2 (10%−12%). This muscle mass-sparing effect is perhaps not surprising considering single-mode AT does not tend to elicit muscle hypertrophy as effectively as RT,44–48 especially in older adults with blunted anabolic response.
Our preliminary data agree with prior literature about the superior ability of WL+RT to improve the ratio of muscle to intermuscular fat lost during weight loss.43 Findings herein also further current knowledge by suggesting that exercise, especially RT, may be able to improve muscle radio-attenuation as measured by CT (indicating less intramuscular fat) in direct comparison to WL alone. These effects are being measured even following a weight maintenance phase (months 12–18), highlighting the relevance of adding exercise to behavioral weight loss programs. The ratio of muscle to intermuscular fat area lost at the mid-thigh was 0.2:1 for WL+RT, compared with 2:1 for both WL and WL+AT. At the mid-thigh, radio-attenuation improvements were more than three times greater in WL+RT than in WL+AT (+0.70 vs +0.19 HU), although this finding must be validated with an appropriate sample size as between group differences were not statistically significant. At the trunk, modest improvements in muscle radio-attenuation were two-fold greater in WL+AT than in WL+RT (+0.68 vs +0.34 HU) and the ratio of muscle to fat lost was 2:1 for WL+RT, compared with 3:1 for both WL and WL+AT.
Effect sizes suggest that adding either AT or RT to WL preserves or slightly improves muscle quality during weight loss, as measured by muscle attenuation and intermuscular fat percentage. As to why WL+AT might have improved muscle attenuation (a measure of intramyocellular lipid content) more at the trunk and WL+RT more at the mid-thigh, one factor to consider is the site-specific nature of exercise. The machine-based RT intervention in this study included six lower extremity exercises (leg press, hip adduction, hip abduction, calf extension, leg extension, and leg curl) and two lumbar trunk-specific exercises (rotary torso and abdominal crunch), which may have influenced the dose response of RT on each body region. Another possibility is that further muscle quality improvements could have been blunted by insufficient protein intake, and therefore making the AT and RT groups more similar than they would have otherwise been. Protein intake for CLIPII was set at a minimum of 0.8 g/kg of body weight per day, with a counseled goal of 1.0g/kg/day, but more recent guidelines suggest active older adults may need at a minimum 1.2 g/kg/day.49 Nevertheless, the protein intake counseled for this study is representative of older adult protein intake in the United States and is in line with the community weight loss setting.50
Other studies support the notion that exercise can improve muscle radio-attenuation.51–53 However, exactly what magnitude change in muscle radio-attenuation is clinically meaningful is unclear. It has been observed cross-sectionally that people living with pathologies related to aging such as type 2 diabetes, obesity, and lower back pain, have lower muscle radio-attenuation by 3–15 HU when compared to participants with no obesity and absent/mild lower back pain.54,55 In an interventional study of patients with low back pain plus disc degeneration and postlaminectomy syndrome, participants undergoing lumbar fusion experienced a decline of 6 HU at the lumbar spine after one year, while patients randomized to exercise and cognitive intervention did not experience any declines.56 Additionally, data from cross-sectional studies suggest that older adults who are overweight have a muscle radio-attenuation 2.5 to 6 HU lower with each decade of life,57,58 although estimates vary between reports. Furthermore, randomized studies of the effect of exercise and diet-induced weight loss on muscle radio-attenuation are lacking. Two studies have reported that older adults (65–83 years and 67–98 years) doing RT with no WL during 10–12 weeks increased their mid-thigh muscle radio-attenuation ~2 HU.51,53 Also, older adults (76–77 years) randomized to one year of light physical activity (1.4% weight reduction) were able to preserve their muscle radio-attenuation, whereas health-education controls experienced a decrease of ~1.4 HU.52 Finally, taken together with the findings herein, these data suggest that pathological processess may be the factor most influential to muscle radio-attenuation, that RT may be most beneficial, and that RT can expect to aim for attenuated losses, preservation, or even modest increases in muscle attenuation during weight-loss. Given that muscle radio-attenuation has been associated with muscle strength,56,59 balance,60 mortality,61,62 and fall and fracture incidence,16 investigating this association further is recommended and future appropriately powered studies with sufficient follow-up time are needed.
Low-trauma fractures are prevalent among patients with low to normal aBMD,63 suggesting that many aspects of bone health are not being captured by traditional osteoporosis screening and that additional independent predictors and state-of-the-art techniques need to be incorporated to design and evaluate effective bone-sparing weight loss strategies. Exploratory analyses presented here suggest that there could be a positive site-specific association between changes in muscle radio-attenuation, and changes in bone strength (r=0.37–0.41) during weight loss, although this association was only significant for the trunk region. We found no association between changes in muscle area and changes in vBMD or bone strength. These findings partially contrast with previous cross-sectional studies that have reported that lower thigh muscle area and radio-attenuation are independently associated with low femoral neck BMD64 and incident hip fracture in ages 70+,15,65 although agreement is not absolute.66 Further research with long follow-up times are needed to fully characterize this muscle-bone association, especially considering differences in the remodeling speed of both tissues and the metabolically dynamic environment that exercise and weight loss interventions create. If future longitudinal studies confirm the association between muscle radio-attenuation and bone strengths, then improvements in muscle quality (for example, via exercise training) could help mitigate declines in bone strength during weight loss in older adults.
Our results should be interpreted considering extensive study limitations. First, we did not evaluate the combination of AT+RT. In Waters et al., it was shown that a combined intervention of aerobic plus resistance training during weight loss resulted in an additive effect of the benefits of each modality and improves visceral adipose tissue, thigh intermuscular adipose tissue, and gait speed more than either exercise modality alone in a population of older adults with frailty.67 However, when a single exercise modality must be adopted, the findings in this study and in Waters et al.67 show that there was no significant difference between the effects of AT and RT in ectopic fat depot variables and that RT significantly spared the most muscle; therefore RT can be confidently prescribed in this regard. Second, there is previous evidence in older adults (70–80 years old) with limited mobility that supplementation (primarily protein) improves ectopic mid-thigh fat measures beyond the effects of physical activity alone18 and may preserve DXA BMD during weight loss68 but our current study did not explore this dietary quality component that may mediate the effects of exercise on muscle and bone. This ancillary study is exploratory in nature and by design, so the preliminary intervention effects found herein should be examined further and replicated in an appropriately powered confirmatory clinical trial. We cannot infer causal relationships, and the 38% loss to follow-up may have led to survivorship bias. The strengths of this study include the evaluation of bone and muscle outcomes, the RCT design, the direct comparison of different exercise modalities with a weight loss only group during community-based weight loss, a relevant sample of older adults with obesity, CVD, or metabolic syndrome, and an 18-month intervention duration.
Finally, we wish to emphasize the importance of assessing multiple musculoskeletal regions comprehensively to maximize the translation of research findings. The use of the thigh region is more established in exercise research, but the trunk region has gained traction in other fields of research such as cachexia and sarcopenia partly because it is more frequently imaged in clinical CT exams (i.e., from cancer screening and follow-up, trauma evaluation, acute pain, etc.).69 Clarification of how exercise and/or weight loss modifies each muscle property is important to translate findings and reach agreement across studies. Correspondingly, a recent meta-analysis highlighted that an impediment to the interpretation of the effects of exercise on muscle quality in older adults is the heterogeneity of sites assessed and partial reports of muscle health.70 In response to this gap, here we reported both muscle radio-attenuation and intermuscular fat area/percentage, and we found different intervention responses and effect sizes for these two “fat infiltration” measures. Intermuscular fat measures the fat depots between muscle fibers, while intramuscular fat is thought to indirectly measure intramyocellular fat content. From the bone health standpoint, BMD and bone strength often display site-heterogeneity, and both the hip and spine are regions of interest as they are common fracture sites associated with elevated morbidity.71,72 Therefore, when possible, reporting comprehensive muscle metrics would optimize efforts towards consensus in musculoskeletal research.
In conclusion, this exploratory analysis suggests that in older adults living with obesity and either cardiovascular disease or metabolic syndrome, WL+RT may be more effective than WL and WL+AT at mitigating mid-thigh muscle area loss. Secondary analyses suggest that changes in muscle radio-attenuation are associated with changes in bone strength at the trunk. We recommend further study to elucidate whether causality can be inferred and to determine the underlying mechanism of this suggestive link.
Supplementary Material
Highlights.
WL+RT better preserved muscle area and improved muscle quality more consistently than WL+AT or WL alone.
Exercise, either RT or AT, may be able to improve muscle radio-attenuation as measured by CT (indicating less intramuscular fat) in direct comparison to WL alone.
Exploratory analyses presented here suggest that there could be a positive site-specific association between changes in muscle radio-attenuation and changes in bone strength during weight loss at the trunk.
More research is needed to characterize the associations between muscle and bone quality in older adults undertaking weight loss interventions and to determine if there is a causal link.
Acknowledgments:
We wish to acknowledge Beverly Nesbit, Jillian Gaukstern and Jessica Sheedy for their contributions related to the supervision and conduct of CLIP-II, and Katelyn Greene for providing training in the segmentation method. We thank all the CLIP-II trial participants.
Funding:
This work was supported by a grant awarded by the National Heart, Lung, Blood Institute to WJR and APM (grant number R18 HL076441). This work was also supported by the National Institutes on Aging grants awarded to the Claude D. Pepper Older Americans Independence Center at Wake Forest School of Medicine (grant number P30 AG021332), KMB (grant number K01 AG047921), and AAW (grant number K25 AG058804). We are indebted to the Fulbright Foreign Student Program for funding DAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Roles
Diana A. Madrid: Conceptualization, Methodology, Software, Formal Analysis, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization
Kristen M. Beavers: Conceptualization, Methodology, Validation, Investigation, Writing - Original Draft, Writing - Review & Editing, Supervision, Project Administration, Funding Acquisition
Michael P. Walkup: Methodology, Software, Formal Analysis, Investigation, Writing - Original Draft, Writing - Review & Editing
Walter T. Ambrosius: Methodology, Writing - Original Draft
W. Jack Rejeski: Conceptualization, Validation, Investigation, Writing - Original Draft, Supervision, Project Administration, Funding Acquisition
Anthony P. Marsh: Conceptualization, Investigation, Writing - Original Draft, Supervision, Project Administration, Funding Acquisition
Ashley A. Weaver: Conceptualization, Methodology, Validation, Writing - Original Draft, Writing - Review & Editing, Supervision, Project Administration, Funding Acquisition
Disclosure: The authors declare no relevant conflicts of interest. Data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.U.S. Department of Health and Human Services. 2017. Profile of Older Americans. Administration for Community Living. Accessed 3/30/2022, https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2017OlderAmericansProfile.pdf
- 2.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3 [DOI] [PubMed] [Google Scholar]
- 3.Gill LE, Bartels SJ, Batsis JA. Weight Management in Older Adults. Curr Obes Rep. Sep 2015;4(3):379–88. doi: 10.1007/s13679-015-0161-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert SM, Venditti EM, Boudreau RM, et al. Weight Loss through Lifestyle Intervention Improves Mobility in Older Adults. The Gerontologist. 2021;doi: 10.1093/geront/gnab048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ades PA, Savage PD. Potential Benefits of Weight Loss in Coronary Heart Disease. Prog Cardiovasc Dis. 2014;56(4):448–456. doi: 10.1016/j.pcad.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 6.Jiang BC, Villareal DT. Weight Loss-Induced Reduction of Bone Mineral Density in Older Adults with Obesity. J Nutr Gerontol Geriatr. Jan-Mar 2019;38(1):100–114. doi: 10.1080/21551197.2018.1564721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papageorgiou M, Kerschan-Schindl K, Sathyapalan T, Pietschmann P. Is Weight Loss Harmful for Skeletal Health in Obese Older Adults? Gerontology. 2020;66(1):2–14. doi: 10.1159/000500779 [DOI] [PubMed] [Google Scholar]
- 8.Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD. Calorie restriction in overweight older adults: Do benefits exceed potential risks? Exp Gerontol. Dec 15 2016;86:4–13. doi: 10.1016/j.exger.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wherry SJ, Miller RM, Jeong SH, Beavers KM. The Ability of Exercise to Mitigate Caloric Restriction-Induced Bone Loss in Older Adults: A Structured Review of RCTs and Narrative Review of Exercise-Induced Changes in Bone Biomarkers. Nutrients. 2021;13(4):1250. doi: 10.3390/nu13041250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med. 2017;376(20):1943–1955. doi: 10.1056/NEJMoa1616338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beavers KM, Walkup MP, Weaver AA, et al. Effect of Exercise Modality During Weight Loss on Bone Health in Older Adults With Obesity and Cardiovascular Disease or Metabolic Syndrome: A Randomized Controlled Trial. J Bone Miner Res. Dec 2018;33(12):2140–2149. doi: 10.1002/jbmr.3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalof H, Dimitri P, Shuweihdi F, Offiah AC. “Which skeletal imaging modality is best for assessing bone health in children and young adults compared to DXA? A systematic review and meta-analysis”. Bone. 2021/09/01/ 2021;150:116013. doi: 10.1016/j.bone.2021.116013 [DOI] [PubMed] [Google Scholar]
- 13.Beavers KM, Beavers DP, Houston DK, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97(3):552–60. doi: 10.3945/ajcn.112.047860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324 [DOI] [PubMed] [Google Scholar]
- 15.Lang T, Cauley JA, Tylavsky F, et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25(3):513–519. doi: 10.1359/jbmr.090807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott D, Johansson J, McMillan LB, Ebeling PR, Nordstrom A, Nordstrom P. Mid-calf skeletal muscle density and its associations with physical activity, bone health and incident 12-month falls in older adults: The Healthy Ageing Initiative. Bone. 2019/03/01/ 2019;120:446–451. doi: 10.1016/j.bone.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 17.Prado CM, Purcell SA, Alish C, et al. Implications of low muscle mass across the continuum of care: a narrative review. Ann Med. 2018/11/17 2018;50(8):675–693. doi: 10.1080/07853890.2018.1511918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Englund DA, Kirn DR, Koochek A, et al. Nutritional Supplementation With Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, The VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J Gerontol A Biol Sci Med Sci. 2017;73(1):95–101. doi: 10.1093/gerona/glx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schileo E, Taddei F. Finite Element Assessment of Bone Fragility from Clinical Images. Curr Osteoporos Rep. 2021/12/01 2021;19(6):688–698. doi: 10.1007/s11914-021-00714-7 [DOI] [PubMed] [Google Scholar]
- 20.Patel HP, Dawson A, Westbury LD, et al. Muscle Mass, Muscle Morphology and Bone Health Among Community-Dwelling Older Men: Findings from the Hertfordshire Sarcopenia Study (HSS). Calcified tissue international. 2018;103(1):35–43. doi: 10.1007/s00223-018-0388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott D, Shore-Lorenti C, McMillan L, et al. Associations of components of sarcopenic obesity with bone health and balance in older adults. Archives of Gerontology and Geriatrics. 2018/03/01/ 2018;75:125–131. doi: 10.1016/j.archger.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 22.Yang Q, Yan D, Wang L, et al. Muscle fat infiltration but not muscle cross-sectional area is independently associated with bone mineral density at the lumbar spine. The British Journal of Radiology. 2022;95(1134):20210371. doi: 10.1259/bjr.20210371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalhoub D, Boudreau R, Greenspan S, et al. Associations Between Lean Mass, Muscle Strength and Power, and Skeletal Size, Density and Strength in Older Men. Journal of Bone and Mineral Research. 2018;33(9):1612–1621. doi: 10.1002/jbmr.3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. Oct 2016;5(10):1042–1047. doi: 10.1016/j.molmet.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dankbar B, Fennen M, Brunert D, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. Sep 2015;21(9):1085–90. doi: 10.1038/nm.3917 [DOI] [PubMed] [Google Scholar]
- 26.Wasgewatte Wijesinghe DK, Mackie EJ, Pagel CN. Normal inflammation and regeneration of muscle following injury require osteopontin from both muscle and non-muscle cells. Skeletal Muscle. 2019/02/26 2019;9(1):6. doi: 10.1186/s13395-019-0190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estell EG, Le PT, Vegting Y, et al. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. eLife. 2020/08/11 2020;9:e58172. doi: 10.7554/eLife.58172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beavers KM, Ambrosius WT, Rejeski WJ, et al. Effect of Exercise Type During Intentional Weight Loss on Body Composition in Older Adults with Obesity. Obesity. 2017;25(11):1823–1829. doi: 10.1002/oby.21977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoell SL, Beavers KM, Beavers DP, et al. Prediction of lumbar vertebral body compressive strength of overweight and obese older adults using morphed subject-specific finite-element models to evaluate the effects of weight loss. Aging Clin Exp Res. 2019;31(4):491–501. doi: 10.1007/s40520-018-1010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoell SL, Weaver AA, Beavers DP, et al. Development of Subject-Specific Proximal Femur Finite Element Models Of Older Adults with Obesity to Evaluate the Effects of Weight Loss on Bone Strength. J Osteoporos Phys Act. 2018;6(1):213. doi: 10.4172/2329-9509.1000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh AP, Janssen JA, Ambrosius WT, et al. The Cooperative Lifestyle Intervention Program-II (CLIP-II): Design and methods. Contemp Clin Trials. 2013/11/01/ 2013;36(2):382–393. doi: 10.1016/j.cct.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rejeski WJ, Ambrosius WT, Burdette JH, Walkup MP, Marsh AP. Community Weight Loss to Combat Obesity and Disability in At-Risk Older Adults. J Gerontol A Biol Sci Med Sci. Oct 12 2017;72(11):1547–1553. doi: 10.1093/gerona/glw252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweitzer L, Geisler C, Pourhassan M, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr. Jul 2015;102(1):58–65. doi: 10.3945/ajcn.115.111203 [DOI] [PubMed] [Google Scholar]
- 34.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 35.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keaveny TM, Clarke BL, Cosman F, et al. Biomechanical Computed Tomography analysis (BCT) for clinical assessment of osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2020;31(6):1025–1048. doi: 10.1007/s00198-020-05384-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–122. doi: 10.1152/jappl.1998.85.1.115 [DOI] [PubMed] [Google Scholar]
- 38.Shen W, Wang Z, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11(1):5–16. doi: 10.1038/oby.2003.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene KA, Withers SS, Lenchik L, Tooze JA, Weaver AA. Trunk Skeletal Muscle Changes on CT with Long-Duration Spaceflight. Ann Biomed Eng. 2021:1–10. doi: 10.1007/s10439-021-02745-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998/04// 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520 [DOI] [PubMed] [Google Scholar]
- 41.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. Jul 2008;9(7):629–35. doi: 10.1016/s1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 42.Altai Z, Qasim M, Li X, Viceconti M. The effect of boundary and loading conditions on patient classification using finite element predicted risk of fracture. Clinical Biomechanics. 2019/08/01/ 2019;68:137–143. doi: 10.1016/j.clinbiomech.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 43.Waters DL, Aguirre L, Gurney AB, et al. Effect of Aerobic or Resistance Exercise, or Both, on Intermuscular and Visceral Fat and Physical and Metabolic Function in Older Adults with Obesity While Dieting. J Gerontol A Biol Sci Med Sci 2021;doi: 10.1093/gerona/glab111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grgic J, McLlvenna LC, Fyfe JJ, et al. Does Aerobic Training Promote the Same Skeletal Muscle Hypertrophy as Resistance Training? A Systematic Review and Meta-Analysis. Sports Medicine. 2019/02/01 2019;49(2):233–254. doi: 10.1007/s40279-018-1008-z [DOI] [PubMed] [Google Scholar]
- 45.Harber MP, Konopka AR, Undem MK, et al. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol (1985). Nov 2012;113(9):1495–504. doi: 10.1152/japplphysiol.00786.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harber MP, Konopka AR, Douglass MD, et al. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. Nov 2009;297(5):R1452–9. doi: 10.1152/ajpregu.00354.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz RS, Shuman WP, Larson V, et al. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism. 1991/05/01/ 1991;40(5):545–551. doi: 10.1016/0026-0495(91)90239-S [DOI] [PubMed] [Google Scholar]
- 48.Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sänger A, Eckstein F. Effect of exercise intervention on thigh muscle volume and anatomical cross-sectional areas—Quantitative assessment using MRI. Magnetic Resonance in Medicine. 2010;64(6):1713–1720. doi: 10.1002/mrm.22550 [DOI] [PubMed] [Google Scholar]
- 49.Bauer J, Biolo G, Cederholm T, et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. Journal of the American Medical Directors Association. 2013/08/01/ 2013;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 50.Krok-Schoen JL, Archdeacon Price A, Luo M, Kelly OJ, Taylor CA. Low Dietary Protein Intakes and Associated Dietary Patterns and Functional Limitations in an Aging Population: A NHANES Analysis. J Nutr Health Aging. 2019/04/01 2019;23(4):338–347. doi: 10.1007/s12603-019-1174-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology. 2009;55(2):217–23. doi: 10.1159/000182084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105(5):1498–1503. doi: 10.1152/japplphysiol.90425.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aas SN, Breit M, Karsrud S, et al. Musculoskeletal adaptations to strength training in frail elderly: a matter of quantity or quality? J Cachexia Sarcopenia Muscle. 2020;11(3):663–677. doi: 10.1002/jcsm.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60(11):1420–4. doi: 10.1093/gerona/60.11.1420 [DOI] [PubMed] [Google Scholar]
- 55.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–110. doi: 10.1152/jappl.2000.89.1.104 [DOI] [PubMed] [Google Scholar]
- 56.Keller A, Brox JI, Gunderson R, Holm I, Friis A, Reikerås O. Trunk Muscle Strength, Cross-sectional Area, and Density in Patients With Chronic Low Back Pain Randomized to Lumbar Fusion or Cognitive Intervention and Exercises. Spine. 2004;29(1):3–8. doi: 10.1097/01.Brs.0000103946.26548.Eb [DOI] [PubMed] [Google Scholar]
- 57.Figueiredo P, Marques EA, Gudnason V, et al. Computed tomography-based skeletal muscle and adipose tissue attenuation: Variations by age, sex, and muscle. Exp Gerontol. 2021;149:111306. doi: 10.1016/j.exger.2021.111306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson DE, D’Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J Gerontol A Biol Sci Med Sci. 2013;68(3):317–23. doi: 10.1093/gerona/gls168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Yin L, Zhao Y, et al. Muscle Density, but Not Size, Correlates Well With Muscle Strength and Physical Performance. J Am Med Dir Assoc. 2020;doi: 10.1016/j.jamda.2020.06.052 [DOI] [PubMed] [Google Scholar]
- 60.Anderson DE, Quinn E, Parker E, et al. Associations of Computed Tomography-Based Trunk Muscle Size and Density With Balance and Falls in Older Adults. J Gerontol A Biol Sci Med Sci. 2015;71(6):811–816. doi: 10.1093/gerona/glv185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boutin RD, Bamrungchart S, Bateni CP, et al. CT of Patients With Hip Fracture: Muscle Size and Attenuation Help Predict Mortality. AJR Am J Roentgenol. 2017;208(6):W208–W215. doi: 10.2214/AJR.16.17226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boutin RD, Katz JR, Chaudhari AJ, et al. Association of adipose tissue and skeletal muscle metrics with overall survival and postoperative complications in soft tissue sarcoma patients: an opportunistic study using computed tomography. Quant Imaging Med Surg. 2020;10(8):1580–1589. doi: 10.21037/qims.2020.02.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lespessailles E, Cortet B, Legrand E, Guggenbuhl P, Roux C. Low-trauma fractures without osteoporosis. Osteoporos Int. 2017;28(6):1771–1778. doi: 10.1007/s00198-017-3921-7 [DOI] [PubMed] [Google Scholar]
- 64.Kim JH, Choi SH, Lim S, et al. Thigh muscle attenuation measured by computed tomography was associated with the risk of low bone density in community-dwelling elderly population. Clin Endocrinol (Oxf). Apr 2013;78(4):512–7. doi: 10.1111/cen.12016 [DOI] [PubMed] [Google Scholar]
- 65.Lang T, Koyama A, Li C, et al. Pelvic body composition measurements by quantitative computed tomography: Association with recent hip fracture. Bone. 2008;42(4):798–805. doi: 10.1016/j.bone.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 66.Reinders I, Murphy RA, Koster A, et al. Muscle Quality and Muscle Fat Infiltration in Relation to Incident Mobility Disability and Gait Speed Decline: the Age, Gene/Environment Susceptibility-Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2015;70(8):1030–1036. doi: 10.1093/gerona/glv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waters DL, Aguirre L, Gurney B, et al. Effect of Aerobic or Resistance Exercise, or Both, on Intermuscular and Visceral Fat and Physical and Metabolic Function in Older Adults With Obesity While Dieting. The Journals of Gerontology: Series A. 2021;doi: 10.1093/gerona/glab111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weaver AA, Houston DK, Shapses SA, et al. Effect of a hypocaloric, nutritionally complete, higher-protein meal plan on bone density and quality in older adults with obesity: a randomized trial. The American Journal of Clinical Nutrition. 2019;109(2):478–486. doi: 10.1093/ajcn/nqy237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mettler FA Jr., Mahesh M, Bhargavan-Chatfield M, et al. Patient Exposure from Radiologic and Nuclear Medicine Procedures in the United States: Procedure Volume and Effective Dose for the Period 2006–2016. Radiology. May 2020;295(2):418–427. doi: 10.1148/radiol.2020192256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radaelli R, Taaffe DR, Newton RU, Galvão DA, Lopez P. Exercise effects on muscle quality in older adults: a systematic review and meta-analysis. Scientific Reports. 2021;11(1):21085. doi: 10.1038/s41598-021-00600-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckstein F, Lochmüller EM, Lill CA, et al. Bone strength at clinically relevant sites displays substantial heterogeneity and is best predicted from site-specific bone densitometry. J Bone Miner Res. Jan 2002;17(1):162–71. doi: 10.1359/jbmr.2002.17.1.162 [DOI] [PubMed] [Google Scholar]
- 72.Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


