Abstract
Pathogen infection causes a stereotyped state of sickness that involves neuronally orchestrated behavioural and physiological changes1,2. On infection, immune cells release a ‘storm’ of cytokines and other mediators, many of which are detected by neurons3,4; yet, the responding neural circuits and neuro–immune interaction mechanisms that evoke sickness behaviour during naturalistic infections remain unclear. Over-the-counter medications such as aspirin and ibuprofen are widely used to alleviate sickness and act by blocking prostaglandin E2 (PGE2) synthesis5. A leading model is that PGE2 crosses the blood–brain barrier and directly engages hypothalamic neurons2. Here, using genetic tools that broadly cover a peripheral sensory neuron atlas, we instead identified a small population of PGE2-detecting glossopharyngeal sensory neurons (petrosal GABRA1 neurons) that are essential for influenza-induced sickness behaviour in mice. Ablating petrosal GABRA1 neurons or targeted knockout of PGE2 receptor 3 (EP3) in these neurons eliminates influenza-induced decreases in food intake, water intake and mobility during early-stage infection and improves survival. Genetically guided anatomical mapping revealed that petrosal GABRA1 neurons project to mucosal regions of the nasopharynx with increased expression of cyclooxygenase-2 after infection, and also display a specific axonal targeting pattern in the brainstem. Together, these findings reveal a primary airway-to-brain sensory pathway that detects locally produced prostaglandins and mediates systemic sickness responses to respiratory virus infection.
Subject terms: Autonomic nervous system, Neuroimmunology
A small population of prostaglandin E2-responsive glossopharyngeal sensory neurons provides a sensory pathway between airway and brainstem that mediates sickness responses to early-phase influenza virus infection.
Main
Respiratory infections caused by influenza and other pathogens are leading causes of death and hospitalization worldwide6, and the recent COVID-19 pandemic has broadly disrupted human society. Feeling sick can be profoundly debilitating, and most people are sick several times a year. The sensation of sickness represents a neural response to infection that may provide a highly coordinated and adaptive strategy to promote recovery1,2. Animals infected with various pathogens display common behavioural and physiological responses that can include fever, lethargy, loss of appetite, headache and pain, mood changes and decreased socialization, suggesting a common sickness state involving shared neural circuits2,7. In addition to common symptoms, other sickness responses are tailored to the site of infection; for example, some respiratory infections induce cough, congestion and bronchoconstriction, whereas some gut infections induce nausea, diarrhoea and vomiting. Infection-specific behavioural responses suggest multiple body–brain communication pathways for pathogen detection.
Several cytokines and immune mediators can induce sickness behaviour when administered in isolation, including interleukins, interferons, tumour necrosis factor (TNF) and eicosanoids1,2,4,8. These and other cytokines, as well as pathogen-derived factors such as lipopolysaccharide, bacterial toxins and formyl peptides, can activate an assortment of central and/or peripheral sensory neurons4,9. Together, these observations raise the possibility that there are many pathways for neuro–immune crosstalk, although it is unclear when or whether each of these pathways is engaged during naturalistic infections.
Chemicals such as salicylic acid from willow bark, aspirin and ibuprofen block biosynthesis of key infection-induced lipid mediators through inhibition of cyclooxygenase enzymes, and have provided a historically effective approach to manage sickness symptoms5,10. Prostaglandin E2 (PGE2) is a key cyclooxygenase-dependent metabolite that evokes sickness behaviour, and knockout of other enzymes downstream of cyclooxygenase in the PGE2 biosynthesis pathway also ameliorates sickness responses11. PGE2 is detected by a small subfamily of 4 G-protein-coupled receptors (EP1–EP4)12, and some but not all pyrogen-induced sickness responses are thought to be mediated by the EP3 receptor2. The EP3 receptor is expressed in various brain regions, including the hypothalamus and circumventricular organs as well as peripheral neurons, immune cells and many other cell types12,13. Region-specific knockout of the EP3 receptor in the median preoptic nucleus (MnPO) of the hypothalamus diminished lipopolysaccharide-induced fever responses14, with PGE2 receptors in other areas reportedly being relevant for effects on arousal and feeding2. These and other findings have led to several possible models in which (1) PGE2 can directly cross the blood–brain barrier owing to its hydrophobicity, (2) PGE2 can be detected or enter the brain at circumventricular organs, and/or (3) PGE2 can be synthesized in the brain itself2,15,16. Central PGE2 could then activate a distributed neural network, with different receptive brain regions such as the MnPO evoking particular aspects of a characteristic sickness response17.
The roles of prostaglandin receptors in peripheral neurons have remained unclear, and furthermore, we reasoned that exogenous application of chemically defined pyrogens may produce a systemic inflammatory response, whereas natural infections in different locations and of varying intensities may instead trigger local neuro–immune response pathways that have remained unexplored.
Influenza causes sickness through EP3
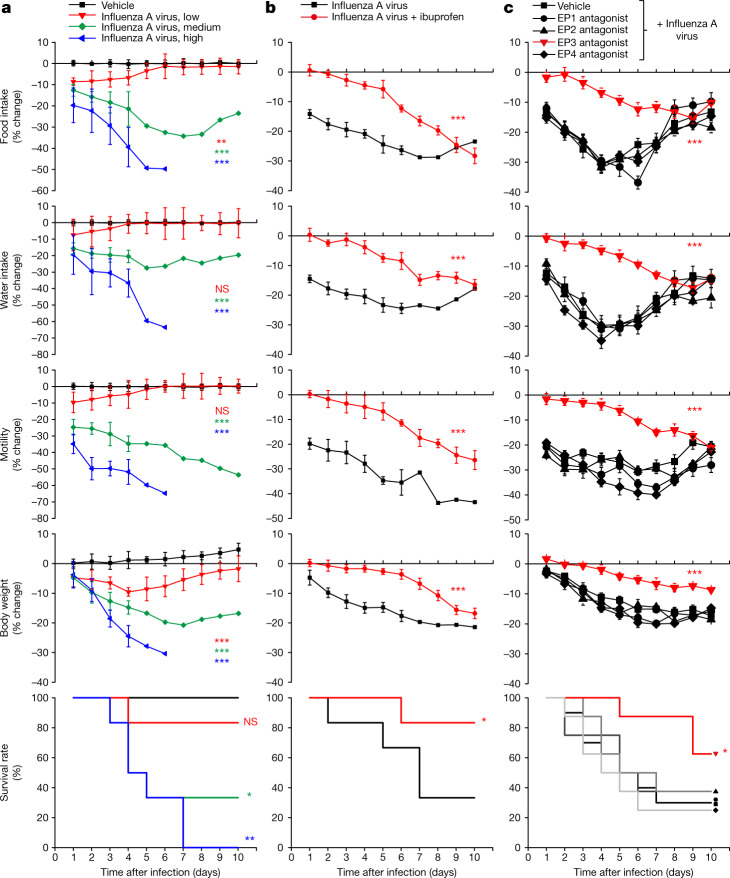
We developed a mouse model to characterize the neuronal mechanisms underlying influenza-induced sickness behaviour. Mice were infected by intranasal administration of influenza A virus PR/8/34 (H1N1), and monitored for characteristic sickness responses over the subsequent 10–20 days. Influenza infection decreased food intake, water intake, locomotion, body weight and survival in wild-type mice. Higher titres of virus inoculum increased the extent of sickness behaviour, with the most severe phenotypes arising six-to-seven days after infection (Fig. 1a). Influenza infection increased PGE2 levels in both plasma and bronchoalveolar lavage fluid (BALF) over a similar time frame, as measured by enzyme-linked immunoassay (ELISA) (Extended Data Fig. 1b). PGE2 administration also acutely inhibited food intake (Extended Data Fig. 2a), and fibre photometry measurements showed decreased activity of hypothalamic neurons expressing agouti-related peptide (AGRP) (Extended Data Fig. 2c), consistent with a decreased motivation to eat, rather than solely a physical inability to eat. We also observed a hypothermic response to influenza infection (Extended Data Fig. 3a), consistent with previous observations in mice18. Administration of ibuprofen (1 mg ml−1 in drinking water, ad libitum) or aspirin (20 mg kg−1, daily intraperitoneal injection) to influenza-infected mice decreased plasma PGE2 levels, restored feeding, water intake and body weight, and promoted survival from infection (Fig. 1b and Extended Data Figs. 1b and 3b). Ibuprofen- and aspirin-treated mice retained low-level decreases in feeding, drinking and motility without elevated PGE2, raising the possibility that other neuro–immune communication pathways might also contribute to the behavioural responses. Yet, ibuprofen and aspirin substantially decreased influenza-induced sickness behaviour, consistent with a key role for cyclooxygenase-2 metabolites in neuro–immune crosstalk.
Fig. 1. Influenza infection induces multiple sickness symptoms through the EP3 receptor.
a, Mice were infected intranasally with 25 μl of influenza A virus at low (105 EID50 ml−1), medium (106 EID50 ml−1) or high (107 EID50 ml−1) dose and food intake, water intake, motility and body weight were subsequently monitored daily. EID50 is the 50% egg infective dose. Data are mean ± s.e.m.; n = 6 mice per group. One-way ANOVA with Dunnett’s multiple comparison test compared with vehicle control. Food intake: P = 0.0048 (low), P < 0.0001 (medium), P < 0.0001 (high). Water intake: P = 0.8185 (low), P < 0.0001 (medium), P < 0.0001 (high). Motility: P = 0.5231 (low), P < 0.0001 (medium), P < 0.0001 (high). Body weight: P = 0.0002 (low), P < 0.0001 (medium), P < 0.0001 (high). Survival: P = 0.3173 (low), P = 0.0185 (medium), P = 0.0007 (high). b, Mice were infected with influenza A virus (all infections are with 106 EID50 ml−1 unless otherwise indicated), given drinking water with or without 1 mg ml−1 ibuprofen, and monitored as indicated. Data are mean ± s.e.m.; n = 6 mice per group. Food intake: P < 0.0001; water intake: P = 0.0001; motility: P = 0.0001; body weight: P < 0.0001; survival: P = 0.0295. c, Mice were infected with influenza A virus, injected daily (intraperitoneal injection, 1 mg kg−1) with antagonists for EP1 (SC-51322), EP2 (PF-04418948), EP3 (DG-041) or EP4 (ONO-AE3-208), or vehicle alone, and monitored as indicated. Data are mean ± s.e.m.; n = 10 mice for vehicle groups; n = 8 mice for all others. For EP3 antagonist—food intake: P < 0.0001; water intake: P < 0.0001; motility: P < 0.0001; body weight: P = 0.0002; survival: P = 0.0094. b,c, Two-tailed unpaired t-test, with comparisons between groups treated with ibuprofen or EP3 antagonist and vehicle in c. Log-rank (Mantel–Cox) test for survival analyses; for behavioural or physiological changes, a mean daily change in behaviour (days 1–10 after infection or survival) was obtained for each mouse, and then used for comparisons across experimental groups (for more information see Extended Data Fig. 1a). *P < 0.05, **P < 0.005, ***P<0.0005; NS, not significant.
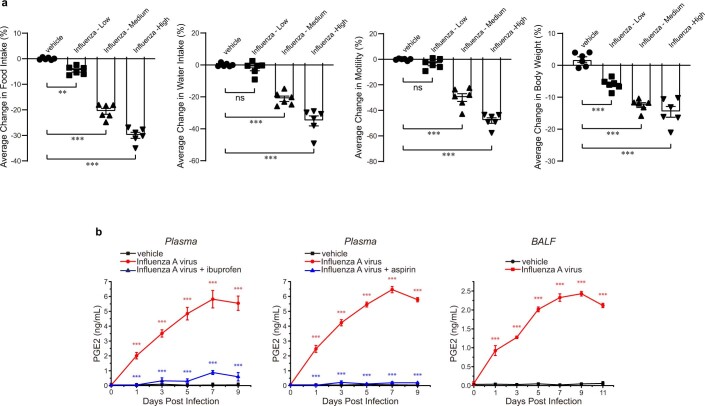
Extended Data Fig. 1. Influenza A infection induces behavioral changes and PGE2 production.
a, Statistical analysis for behavioral experiments involved averaging daily changes in parameters indicated per mouse (days 1-10 after infection or through survival), as represented here in bar graphs. Statistical values are identical to those in Fig. 1a. b, PGE2 levels in plasma (left, middle) and BALF (right) were measured by ELISA at time points indicated after exposure to influenza A virus (red, blue) or vehicle control (black). Some virus-infected mice (blue) were additionally given ad libitum access to ibuprofen (1 mg/ml) in drinking water from 3 days prior to infection (left) or received daily aspirin administration (IP, 20 mg/kg), mean ± sem, n: 3 mice per group, ***p < 0.0005 by two-way ANOVA followed by Bonferroni’s multiple comparison test with comparisons made between red and black curves (red stars) or red and blue curves (blue stars). p values in b are <0.0001 for all indicated stars.
Extended Data Fig. 2. PGE2 decreases feeding and AGRP neuron activity.
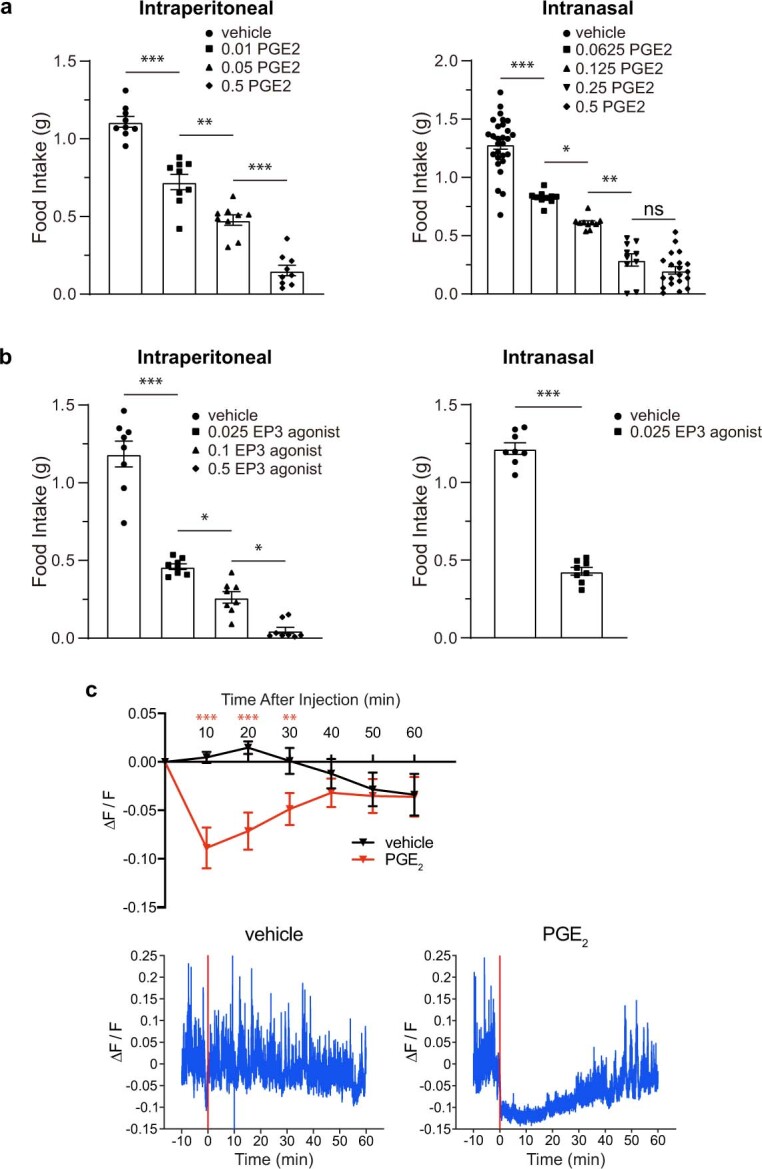
a, Food intake by fasted mice administered with PGE2 (left: IP, right: intranasal) at doses indicated (mg/kg), and given ad libitum access to food (1 h), mean ± sem, n: 9 (left) mice per group, 28 (vehicle), 10 (0.0625, 0.125, 0.25), 20 (0.5) in (right), ***p < 0.0005, **p < 0.005, *p < 0.05, ns: not significant by two-way ANOVA Tukey’s multiple comparison test. b, Food intake by fasted mice administered with the EP3 receptor agonist sulprostone (left: IP, right: intranasal) at doses indicated (mg/kg), and given ad libitum access to food (1 h), mean ± sem, n: 8 mice per group, ***p < 0.0005, *p < 0.05 by two-way ANOVA Tukey’s multiple comparison test (left) or two-tailed unpaired t-test (right). c, GCaMP6s fluorescence (ΔF/F) was measured in AGRP neurons of the arcuate nucleus by fiber photometry before and after IP injection of PGE2 (0.5 mg/kg in PBS) or vehicle alone (PBS). (top) Responses are depicted as the mean of measurements made in 10-minute time intervals (for example, 10 refers to the mean of measurements made between 0 and 10 min), mean ± sem, n: 12 mice per group, **p < 0.01, ***p < 0.001 by two-way ANOVA with Bonferroni’s multiple comparison test, (bottom) representative recording traces with red bar indicating time of injection. p values left to right in a: IP: <0.0001, 0.0005, <0.0001, intranasal: <0.0001, 0.0455, 0.0008, 0.6630; b, IP: <0.0001, 0.0312, 0.0187, intranasal: <0.0001; c: <0.0001, <0.0001, 0.0045.
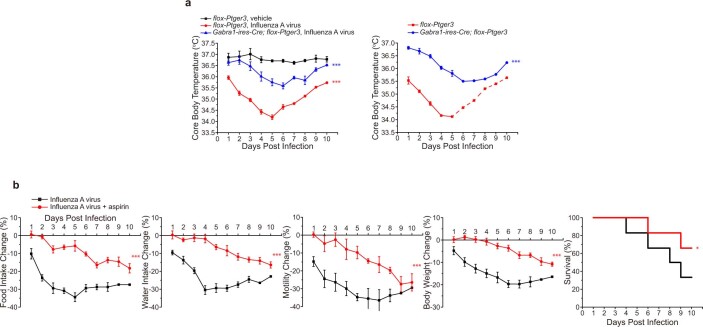
Extended Data Fig. 3. Influenza-induced hypothermia and sickness behaviors are attenuated by cell-specific Ptger3 knockout and aspirin.
a, Core body temperature in flox-Ptger3 and Gabra1-ires-Cre; flox-Ptger3 mice was measured daily after administration of influenza virus (red, blue) or saline (black) using a rectal probe (left) or radiotelemetry (right), mean ± sem, n: 6 (left) and 3 (right) mice per group, ***p < 0.0005 by one-way ANOVA Dunnett’s multiple comparison test (left); ***p < 0.0005 by two-tailed unpaired t-test for right as detailed in Fig. 1 for behavior/physiology analysis (right), comparisons between red and black curves (red stars) or between red and blue curves (blue stars). For data on the right, two of three control flox-Ptger3 mice died on day 5, so subsequent data is represented as a dashed line and statistical analysis was performed only on data from days 1-4. b, Mice were given daily injections of aspirin (IP, 20 mg/kg), red or vehicle alone (black) throughout the paradigm. After three days, mice were infected with influenza A virus and subsequently monitored as indicated, mean ± sem, n: 6 mice per group, ***p < 0.0005 by two-tailed unpaired t-test as detailed in Fig. 1 for behavior/physiology analysis, *p < 0.05 by a log-rank (Mantel-Cox) test for survival analysis. p values in a, left: red <0.0001, blue 0.0003, right: <0.0001; b, left to right: <0.0001, <0.0001, <0.0001, <0.0001, 0.0243.
Roles for multiple prostaglandin receptors have been proposed in sickness behaviours2,19. We administered selective antagonists for each PGE2 receptor daily after influenza infection, and measured different aspects of sickness behaviour. The EP3 receptor antagonist DG-041 effectively blocked influenza-induced sickness in each parameter measured and also promoted survival (Fig. 1c), with an effect magnitude similar to that of ibuprofen and aspirin. By contrast, antagonism of the EP1, EP2 or EP4 receptors had no effect on any measured parameter. The EP3-selective agonist sulprostone had the opposite effect of inhibiting food intake (Extended Data Fig. 2b). Thus, mice display characteristic behavioural changes to influenza virus infection through the action of PGE2 on EP3 receptors.
Glossopharyngeal neurons and sickness
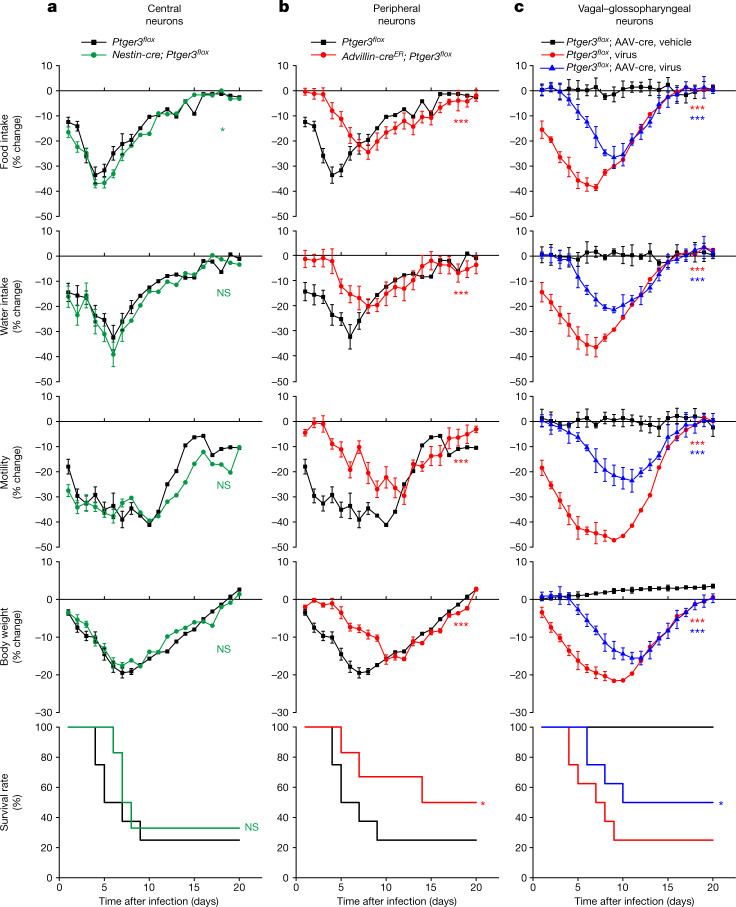
The EP3 receptor is expressed in several classes of central and peripheral neurons. To determine the key site of EP3 receptor action in influenza-induced sickness, we obtained mice with an allele (Ptger3flox) for Cre-dependent knockout of the Ptger3 gene14, which encodes the EP3 receptor. We then crossed Ptger3flox mice with either Nestin-cre or Advillin-creER mice, which target Cre recombinase to most central or peripheral neurons20,21, respectively (Extended Data Fig. 4a), and measured influenza-induced sickness behaviours. Advillin-creER; Ptger3flox mice were treated with tamoxifen to induce Cre-mediated recombination at least one week before virus administration; prior tamoxifen treatment of control mice did not affect the subsequent behavioural responses to influenza infection (Extended Data Fig. 4b). Influenza-induced decreases in feeding, drinking, movement, body weight and survival were attenuated in Advillin-creER; Ptger3flox mice (Fig. 2b), with effect magnitudes similar to ibuprofen treatment, but persisted in Nestin-cre; Ptger3flox mice (Fig. 2a). Similar effects of Ptger3 gene deletion on sickness behaviour were observed when lower, less lethal doses of influenza virus were used (Extended Data Fig. 5). Mice with attenuated sickness behaviour displayed a similar recovery time, as normal feeding, drinking and motility were observed by about two weeks after infection in all experimental groups. These observations indicated that influenza induces sickness responses through PGE2 action on peripheral sensory neurons.
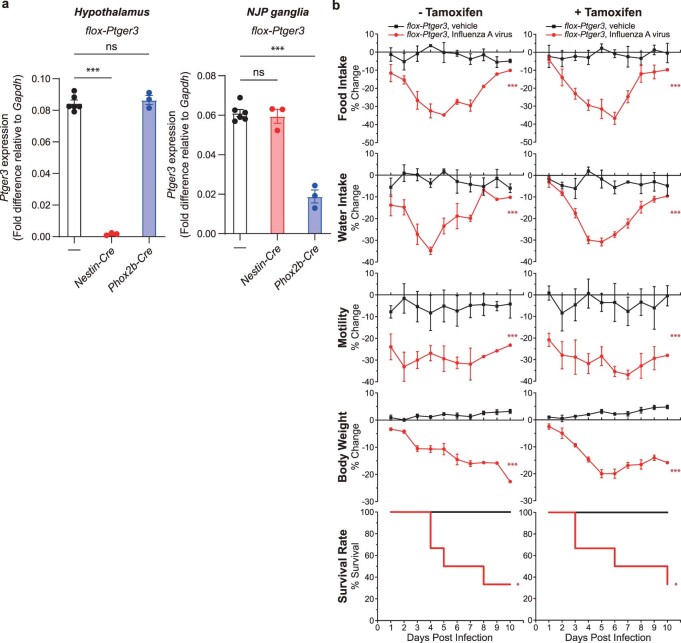
Extended Data Fig. 4. Validating approaches for cell-specific Ptger3 knockout.
a, qPCR analysis of Ptger3 expression in hypothalamus (left) or NJP ganglia (right) of flox-Ptger3 (white), Nestin-Cre; flox-Ptger3 (red), and Phox2b-Cre; flox-Ptger3 (blue) mice. Phox2b-Cre mice display Cre expression in nodose and petrosal but not jugular neurons56. Ptger3 transcript levels were expressed after normalization to Gapdh expression levels, mean ± sem, n: 6 mice for flox-Ptger3 and 3 for other groups, ***p < 0.0005, ns: not significant by one-way ANOVA Dunnett’s multiple comparison test. b, flox-Ptger3 control mice were treated with (right) or without (left) tamoxifen for 5 consecutive days (IP, 70 mg/kg) as was done for Advillin-CreER; flox-Ptger3 mice. At least one week later, mice were either exposed to influenza A virus (red) or saline (black) and monitored as indicated, mean ± sem, n: 6 mice per group, ***p < 0.0005 by two-tailed unpaired t-test as detailed in Fig. 1 for behavior/physiology analysis, *p < 0.05 by a log-rank (Mantel-Cox) test for survival analysis. p values in a, left: <0.0001, 0.7506, right: 0.8680, <0.0001; b top to bottom, left: <0.0001, <0.0001, <0.0001, <0.0001, 0.0179, right: <0.0001, 0.0003, <0.0001, 0.0179.
Fig. 2. Peripheral EP3 receptor is required for influenza-induced sickness.
a,b, Nestin-cre; Ptger3flox (a), Advillin-creER; Ptger3flox mice (b) or Ptger3flox (a,b) mice were infected with influenza A virus and monitored as indicated. Data are mean ± s.e.m.; n = 8 mice (Ptger3flox), n = 6 mice (Nestin-cre and Advillin-creER). Two-tailed unpaired t-test as detailed in Fig. 1 for behaviour or physiology analyses; log-rank (Mantel–Cox) test for survival analysis. a, Food intake: P = 0.0126; water intake: P = 0.1006; motility: P = 0.0701; body weight: P = 0.9361; survival: P = 0.7735. b, Food intake: P = 0.0004; water intake: P = 0.0004; motility: P < 0.0001; body weight: P = 0.0004; survival: P = 0.0216. c, The NJP ganglia of Ptger3flox mice were injected bilaterally with AAV-cre, exposed to influenza A virus or saline and monitored as indicated. Data are mean ± s.e.m.; n = 8 mice per group. Ptger3flox, virus—food intake: P < 0.0001; water intake: P < 0.0001; motility: P < 0.0001; body weight: P < 0.0001; survival: P < 0.0001. Ptger3flox; AAV-cre, virus—Food intake: P < 0.0001; water intake: P < 0.0001; motility: P < 0.0001; body weight: P < 0.0001; survival: P = 0.0376. One-way ANOVA with Dunnett’s multiple comparison test as detailed in Fig. 1 for behaviour or physiology analyses; log-rank (Mantel–Cox) test for survival analysis, with comparisons made between Ptger3flox, virus and Ptger3flox; AAV-cre, vehicle (red stars) or between Ptger3flox, virus and Ptger3flox; AAV-cre, virus (blue stars).
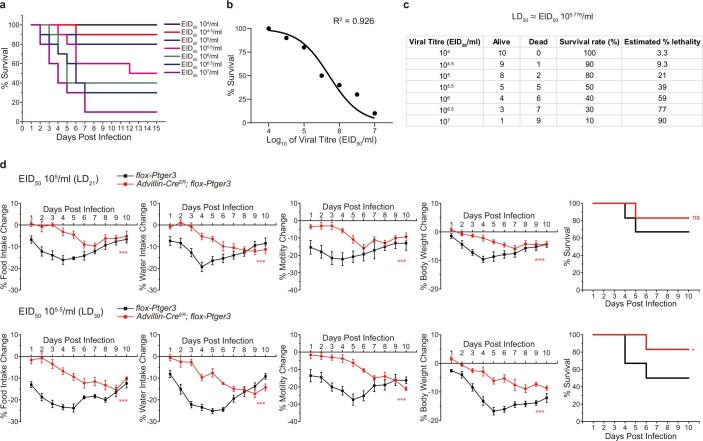
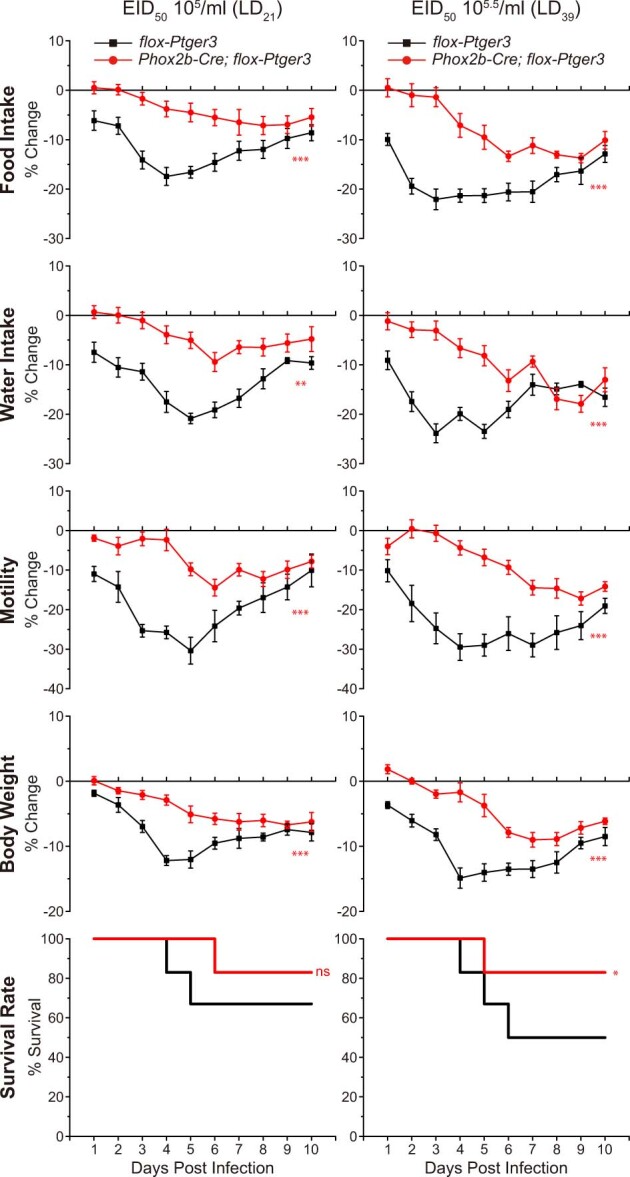
Extended Data Fig. 5. Ptger3 knockout in peripheral sensory neurons attenuates behavioral responses to sublethal influenza A doses.
a, Wild type mice were infected with influenza A virus at titers indicated and subsequent survival monitored daily, n: 10 per group. b, a dose-response curve of viral titer (log10) vs. survival rates with non-linear fit of R2 = 0.926. c, A table indicating survival rates to various influenza A virus inoculation doses, with the estimated % lethality determined by Probit regression analysis (Statistical Product and Service Solutions). d, Advillin-CreER; flox-Ptger3 mice (red, previously injected with tamoxifen) or flox-Ptger3 mice (black) indicated were infected with sublethal doses of influenza A virus (top: 105 EID50 or LD21, bottom: 105.5 EID50 or LD39) and monitored daily as indicated, mean ± sem, n: 6 mice per group, ***p < 0.0005 by two-tailed unpaired t-test as detailed in Fig. 1 for behavior/physiology analysis, *p < 0.05, ns: not significant by a log-rank (Mantel-Cox) test for survival analysis. p values left to right in d, top: <0.0001, <0.0001, 0.0002, <0.0001, 0.4788; bottom: <0.0001, <0.0001, 0.0004, 0.0004, 0.0078.
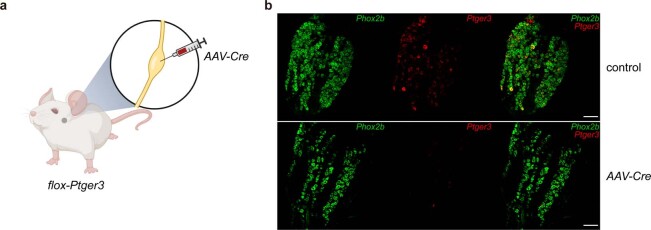
The EP3 receptor is expressed in several classes of peripheral neurons marked in Advillin-creER mice, including vagal sensory neurons, glossopharyngeal sensory neurons, spinal sensory neurons of the dorsal root ganglia, and potentially in other neuron types20,22. Glossopharyngeal and vagal sensory neurons were considered primary candidates for the detection of respiratory pathogens as they account for most of the innervation in the upper and lower airways23. In mice, the soma of vagal (nodose and jugular) and glossopharyngeal (petrosal) sensory neurons are fused into a large superganglion on each side of the body23. We injected an adeno-associated virus (AAV) with a constitutive cre allele (AAV-cre) bilaterally into both nodose–jugular–petrosal (NJP) ganglia of Ptger3flox mice. Effective knockout of Ptger3 in NJP ganglia was confirmed two weeks after AAV injection by RNA in situ hybridization (Extended Data Fig. 6). NJP ganglion-targeted Ptger3 knockout caused a marked attenuation of influenza-induced sickness behaviour (Fig. 2c). These findings indicate that influenza induces behavioural changes through EP3 receptor expressed on vagal and/or glossopharyngeal sensory afferents.
Extended Data Fig. 6. Validating AAV-driven Ptger3 knockout in NJP ganglia.
a, Cartoon depicting bilateral injection of AAV-Cre into NJP ganglia of flox-Ptger3 mice. b, The NJP ganglia of flox-Ptger3 mice were injected bilaterally with AAV-Cre (bottom) or saline (control, top), and cryosections of NJP ganglia were subsequently examined by two-color RNA in situ hybridization to detect Phox2b (green) and Ptger3 (red), scale bar: 100 μm. Images are representative from three independent experiments. Part a created with BioRender.com.
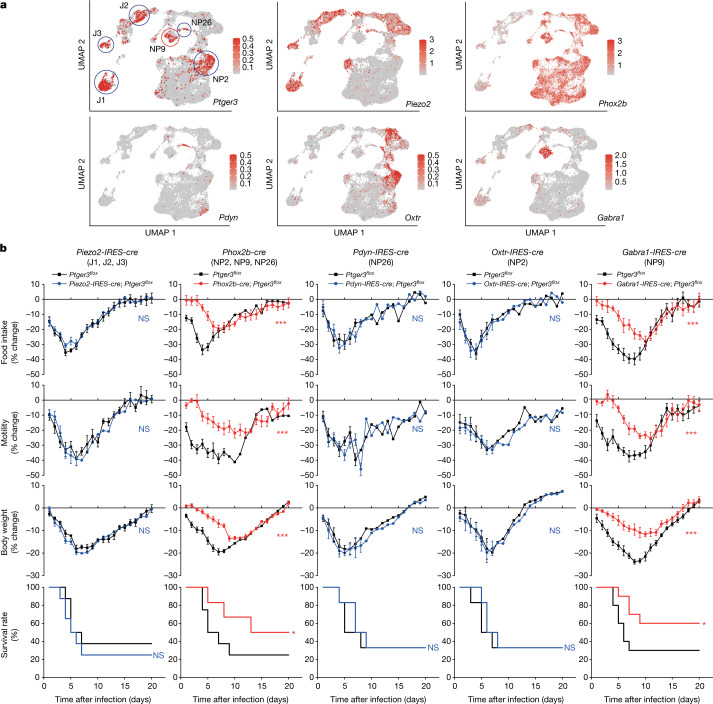
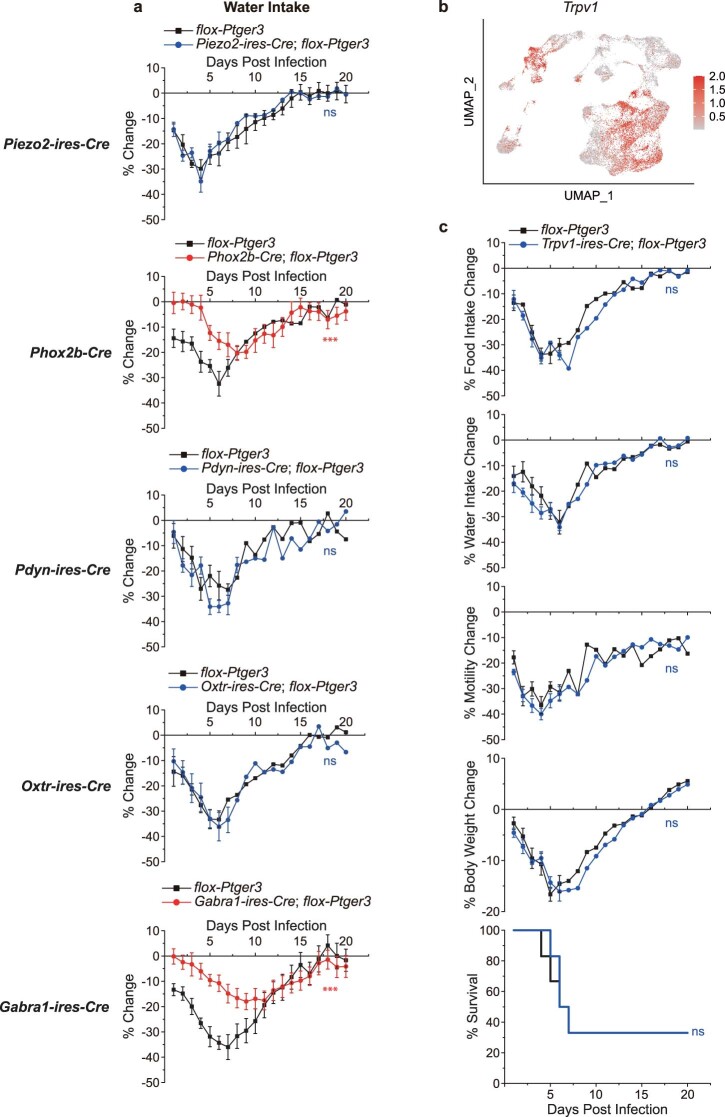
Ptger3 is expressed in a subset of vagal and glossopharyngeal sensory neurons, as revealed by RNA in situ hybridization (Extended Data Fig. 6b) and analysis of single-cell transcriptome data (Fig. 3a). Single-cell RNA sequencing approaches revealed dozens of molecularly distinct NJP sensory neurons22,24,25. The highest Ptger3 expression was observed in 6 neuron clusters: J1, J2, J3, NP2, NP9 and NP26 (Fig. 3a), with J denoting jugular and NP denoting nodose–petrosal neurons. We crossed Ptger3flox mice to Piezo2-IRES-cre mice, in which J1, J2, J3 and other NJP neurons are labelled, and Phox2b-cre mice, in which NP2, NP9, NP26 and other NJP neurons are labelled. Influenza-induced sickness behaviours were attenuated in Phox2b-cre; Ptger3flox mice across a range of viral titres, but not in Piezo2-IRES-cre; Ptger3flox mice (Fig. 3b and Extended Data Figs. 7 and 8a), suggesting a role for either NP2, NP9 or NP26 neurons. To distinguish these neuron types, we next crossed Ptger3flox mice to Pdyn-IRES-cre, Oxtr-IRES-cre and Gabra1-IRES-cre mice, which display differential targeting of NP26, NP2 and NP9 neurons. Gabra1-IRES-cre; Ptger3flox mice displayed a marked attenuation of influenza-induced sickness behaviour similar to ibuprofen treatment, whereas no effect was observed in either Pdyn-IRES-cre; Ptger3flox or Oxtr-IRES-cre; Ptger3flox mice (Fig. 3b and Extended Data Fig. 8a). Gabra1-IRES-cre; Ptger3flox mice additionally displayed an attenuated hypothermia response to influenza infection (Extended Data Fig. 3a). We also tested a role for TRPV1 neurons since their deletion affects survival following bacterial lung infections that cause lethal pneumonia26. However, influenza-induced sickness behaviour was normal in Trpv1-IRES-cre; Ptger3flox mice (Extended Data Fig. 8b,c), and furthermore, GABRA1 neurons are predominantly Trpv1-negative. Thus, deletion of the EP3 receptor in a small subset of Gabra1-expressing peripheral sensory neurons (NP9 neurons) has a wide-ranging effect on behavioural responses to influenza infection.
Fig. 3. Rare transcriptome-defined sensory neurons mediate influenza responses.
a, A uniform manifold approximation and projection (UMAP) plot derived from published single-cell transcriptome data of vagal and glossopharyngeal sensory ganglia22 showing expression of indicated genes (colour shows relative expression on a natural log scale). b, Cell types, as highlighted in a, express the indicated gene as well as Ptger3. Mice were infected with influenza A virus and monitored as indicated. Data are mean ± s.e.m.; n = 8 (Piezo2-IRES-cre), n = 6–8 (Phox2b-cre; 8 control and 6 Phox2b-cre), n = 6 (Pdyn-IRES-cre), n = 6 (Oxtr-IRES-cre) and n = 10 (Gabra1-IRES-cre) mice per group. Two-tailed unpaired t-test as detailed in Fig. 1 for behaviour or physiology analyses; log-rank (Mantel–Cox) test for survival analysis. Piezo2-IRES-cre—food intake: P = 0.2631; motility: P = 0.2680; body weight: P = 0.7925; survival: P = 0.4736. Phox2b-cre—food intake: P < 0.0001; motility: P < 0.0001; body weight: P = 0.0002; survival: P = 0.0181. Pdyn-IRES-cre—food intake: P = 0.4539; motility: P = 0.4946; body weight: P = 0.2675; survival: P = 0.7937; Oxtr-IRES-cre—food intake: P = 0.4786; motility: P = 0.6333; body weight: P = 0.3297; survival: P = 0.7121; Gabra1-IRES-cre—food intake: P = 0.0001; motility: P < 0.0001; body weight: P = 0.0004; survival: P = 0.0263.
Extended Data Fig. 7. Cell-specific Ptger3 knockout attenuates behavioral responses to sublethal influenza A doses.
Phox2b-Cre; flox-Ptger3 mice (red) or flox-Ptger3 mice (black) were infected with sublethal doses of influenza A virus (top: 105 EID50 or LD21, bottom: 105.5 EID50 or LD39) and monitored daily as indicated, mean ± sem, n: 6 mice per group, **p < 0.005, ***p < 0.0005 by two-tailed unpaired t-test as detailed in Fig. 1 for behavior/physiology analysis, *p < 0.05, ns: not significant by a log-rank (Mantel-Cox) test for survival analysis. p values top to bottom left: <0.0001, <0.0001, <0.0001, 0.0002, 0.4524; right: <0.0001, <0.0001, <0.0001, 0.0002, 0.0155.
Extended Data Fig. 8. Measures of flu-induced behavioral changes in cell-specific Ptger3 knockouts.
a, Water intake changes after flu infection in mice of Fig. 3, mean ± sem, n: 8 (Piezo2-ires-Cre), 6-8 (Phox2b-Cre; 8 control and 6 Phox2b-Cre), 6 (Pdyn-ires-Cre), 6 (Oxtr-ires-Cre), and 10 (Gabra1-ires-Cre) mice per group, ***p < 0.0005, ns: not significant by two-tailed unpaired t-test as detailed in Fig. 1 for behavior/physiology analysis. b, A Uniform Manifold Approximation and Projection (UMAP) plot derived from published single-cell transcriptome data of vagal and glossopharyngeal sensory ganglia22 indicating Trpv1 expression (red shading: natural log scale). c, Trpv1-ires-Cre; flox-Ptger3 mice (blue) or flox-Ptger3 mice (black) were infected with influenza A virus and monitored daily as indicated, mean ± sem, n: 6 mice per group, ns: not significant by two-tailed unpaired t-test as detailed in Fig. 1 for behavior/physiology analysis, ns: not significant by a log-rank (Mantel-Cox) test for survival analysis. p values top to bottom in a: 0.9101, 0.0004, 0.0534, 0.9544, <0.0001 and c: 0.2485, 0.0705, 0.0888, 0.1801, 0.8412.
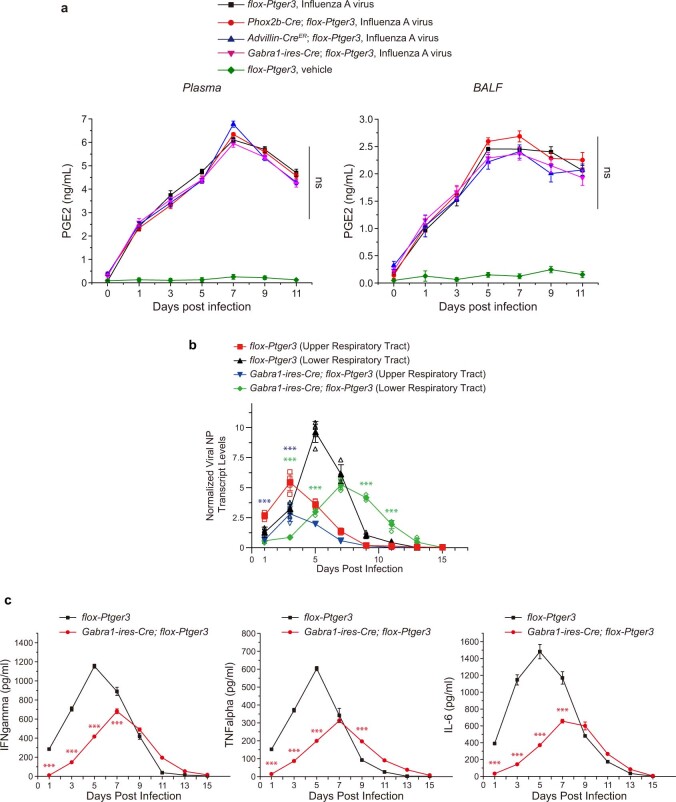
Next, we examined how manipulations of sensory neurons affected viral transcript levels and cytokine production. Influenza infection induced PGE2 to similar levels in plasma and BALF of Gabra1-IRES-cre; Ptger3flox, Phox2b-cre; Ptger3flox, Advillin-creER; Ptger3flox and Ptger3flox mice (Extended Data Fig. 9a), consistent with changes in PGE2 detection rather than PGE2 synthesis underlying the observed behavioural differences. In control mice, viral transcript levels peaked three days after infection in the upper airways and five days after infection in the lungs. In Gabra1-IRES-cre; Ptger3flox mice, viral transcript levels were partially reduced in the upper airways and were decreased, delayed and persistent in the lungs (Extended Data Fig. 9b). Levels of interferon-gamma (IFNγ), TNF and interleukin 6 (IL-6) similarly peaked in BALF of control mice 5 days after infection, and were likewise decreased and delayed in Gabra1-IRES-cre; Ptger3flox mice (Extended Data Fig. 9c). These findings indicate that targeted EP3 receptor knockout in sensory neurons not only affects sickness behaviour, but also the immune response and the transition from upper to lower respiratory tract infection.
Extended Data Fig. 9. Impact of targeted Ptger3 knockout on cytokines and viral transcript levels.
a, PGE2 levels in plasma (left) and BALF (right) were measured by ELISA in mice indicated at various time points after exposure to influenza A virus or PBS control, mean ± sem, n: 5 mice per group, ns: not significant by two-way ANOVA involving analysis of influenza virus-infected groups. b, qPCR analysis of viral nucleoprotein (NP) transcript levels in the upper and lower respiratory tract of flox-Ptger3 and Gabra1-ires-Cre; flox-Ptger3 mice after influenza virus infection, normalized to Gapdh and uninfected controls, mean ± sem, n: 5 mice per group, ***p < 0.0005 by two-way ANOVA followed by Bonferroni’s multiple comparison test with comparisons made between red and blue curves (blue stars) or black and green curves (green stars). c, Levels of IFNγ, TNFα, and IL-6 in BALF were measured by ELISA at time points indicated after exposure to influenza A virus in flox-Ptger3 (black) or Gabra1-ires-Cre; flox-Ptger3 (red), mean ± sem, n: 3 mice per group, ***p < 0.0005 by two-way ANOVA followed by Bonferroni’s multiple comparison test with comparisons made between red and black curves (red stars). p values in a: 0.1591, 0.0662, b and c: <0.0001 for all indicated stars.
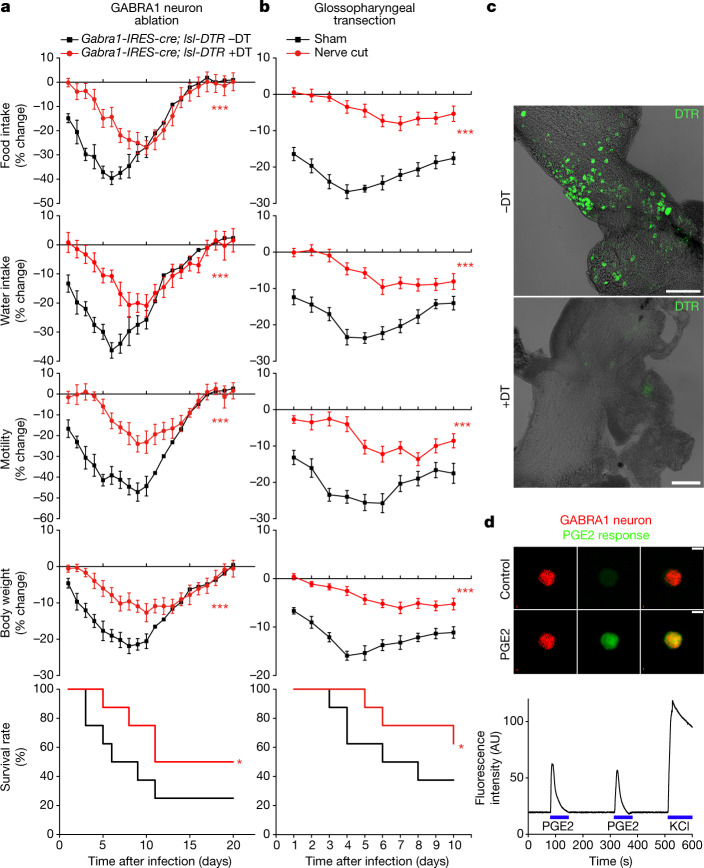
We used a complementary approach involving diphtheria toxin-guided cell ablation to clarify a role for Gabra1-expressing vagal–glossopharyngeal sensory neurons in influenza-induced sickness, and rule out a role for other Gabra1 expression sites. Mouse cells are normally resistant to diphtheria toxin, but can be rendered sensitive by the expression of the human diphtheria toxin receptor (DTR). NJP ganglia of Gabra1-IRES-cre; lsl-DTR mice were bilaterally injected with diphtheria toxin (we term these Gabra1-ABLATE mice), resulting in highly efficient ablation of vagal and glossopharyngeal GABRA1 neurons (Fig. 4c). We previously demonstrated that this approach does not affect Cre-negative neurons in NJP ganglia, or remotely located Cre-positive neurons27. Gabra1-ABLATE mice displayed attenuated sickness responses to influenza infection that were similar to those in Gabra1-IRES-cre; Ptger3flox mice or ibuprofen-treated mice (Fig. 4a).
Fig. 4. PGE2 acts via glossopharyngeal sensory neurons.
a, Gabra1-IRES-cre; lsl-DTR mice were injected bilaterally in NJP ganglia with or without diphtheria toxin (DT) and then infected with influenza A virus and monitored as indicated. Data are mean ± s.e.m.; n = 8 mice per group. Food intake: P < 0.0001; water intake: P < 0.0001; motility: P < 0.0001; body weight: P = 0.0004; survival: P = 0.049. b, Wild-type mice with bilateral glossopharyngeal nerve transection surgery or sham surgery were infected with influenza A virus and monitored as indicated. Data are mean ± s.e.m.; n = 8 mice per group. Food intake: P < 0.0001; water intake: P < 0.0001; motility: P < 0.0001; body weight: P < 0.0001; P = 0.036. Two-tailed unpaired t-test as detailed in Fig. 1 for behaviour or physiology analysis; log-rank (Mantel–Cox) test for survival analysis. c, Immunostaining for DTR in whole-mount preparations of NJP ganglia four weeks after injection. Scale bars, 200 μm. d, Calcium transients evoked by PGE2 (1 μM) or KCl (150 mM) were imaged using Calbryte 520 AM in tdTomato-positive neurons acutely collected from NJP ganglia of Gabra1-IRES-cre; lsl-tdTomato mice. Scale bar, 10 μm. Images are representative of three technical replicates. AU, arbitrary units.
NP9 neurons lack expression of Hoxb4(ref. 22), and GABRA1 neurons are often clustered within NJP ganglia near the glossopharyngeal branch (Extended Data Fig. 10a), suggesting they comprise part of the glossopharyngeal nerve rather than the vagus nerve. Since diphtheria toxin injection similarly affected vagal and glossopharyngeal neurons, we distinguished the contributions from these nerves by transecting the glossopharyngeal nerve bilaterally while preserving the vagus nerve. Mice with bilateral glossopharyngeal nerve transection displayed a similar attenuation of influenza-induced sickness (Fig. 4b). PGE2 was previously shown to activate and/or induce transcriptional changes in spinal and vagal afferents28–31, so we tested whether GABRA1 neurons are also directly activated. We observed that PGE2 directly evoked calcium transients in GABRA1 neurons acutely cultured from NJP sensory ganglia of Gabra1-IRES-cre; lsl-tdTomato mice (Fig. 4d, 16 out of 26 or 61.5% of tdTomato-positive, KCl-responsive neurons). Thus, rare glossopharyngeal NP9 neurons detect the presence of a respiratory viral infection indirectly through the EP3 receptor and, in response, evoke a multi-pronged programme of sickness behaviour.
Extended Data Fig. 10. Sparse innervation of internal organs by GABRA1 NJP neurons.
a, Native GFP fluorescent signals in wholemount preparations of NJP ganglia from Gabra1-ires-cre; lsl-L10 GFP, scale bar: 200 μm. b, NJP ganglia of Gabra1-ires-Cre mice were injected bilaterally with Cre-dependent AAV-flex-AP or AAV-flex-tdTomato and axons were visualized in fixed wholemount tissue preparations using either a colorimetric alkaline phosphatase substrate (top two rows, left images in bottom two rows) or tdTomato immunostaining (right two images in bottom two rows). Scale bars (left to right) top row: 500, 1000, 1000 (inset: 250) μm; 2nd row: 500, 1000, 500 μm; 3rd row: 200, 50 μm; bottom row: 200, 100 μm. Images are representative of three independent experiments involving GFP and tdTomato and two independent experiments involving alkaline phosphatase.
A sensory arc from nasopharynx to brain
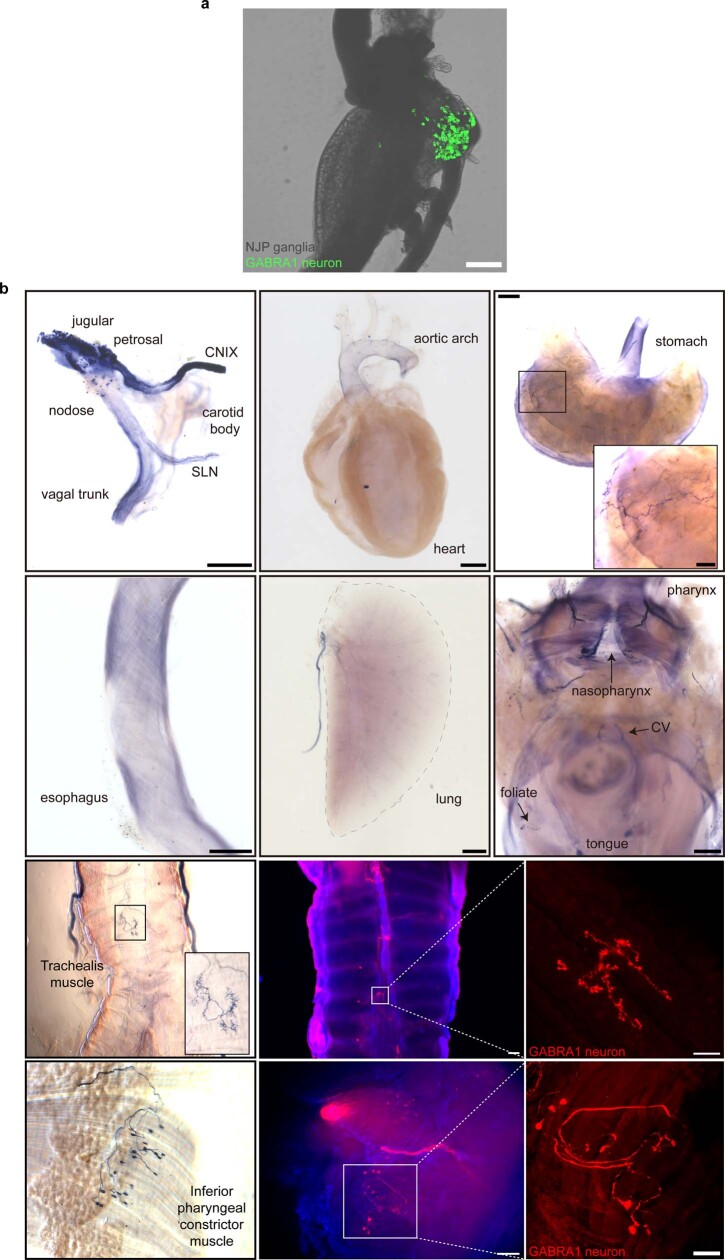
Gabra1-IRES-cre mice provide a valuable tool for visualizing scarce glossopharyngeal sensory neurons involved in neuro–immune crosstalk, so we used genetic mapping approaches to mark the peripheral and central projections of GABRA1 neurons (Fig. 5a). NJP ganglia of Gabra1-IRES-cre mice were injected with either an AAV containing a Cre-dependent reporter gene encoding tdTomato (AAV-flex-tdTomato) or alkaline phosphatase (AAV-flex-AP), as well as an AAV containing a Cre-independent reporter gene encoding GFP (AAV-GFP) to visualize the global NJP projection field. Fibres were then visualized in the airways and the brain.
Fig. 5. GABRA1 neurons provide an airway–brain communication route.
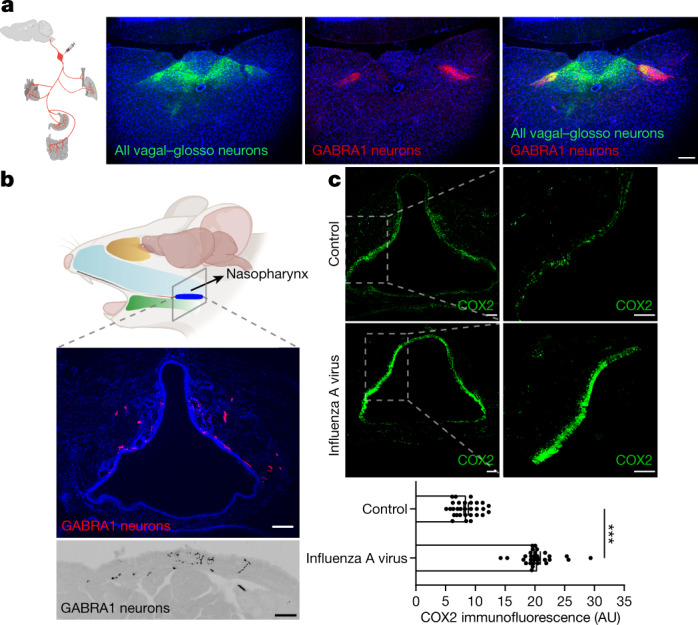
a, NJP ganglia of Gabra1-IRES-cre mice were injected bilaterally with Cre-independent AAV-GFP and Cre-dependent AAV-flex-tdTomato, and fluorescent axons were visualized (green: all NJP sensory axons; red: GABRA1 NJP axons) by immunohistochemistry for tdTomato and GFP in fixed coronal cryosections of mouse brainstem. Scale bar, 200 μm. b, NJP ganglia of Gabra1-IRES-cre mice were injected bilaterally with AAV-flex-tdTomato, and axons were visualized by immunostaining for tdTomato in fixed coronal cryosections of nasopharynx. Scale bars, 100 μm (top), 50 μm (bottom). c, Top, immunohistochemistry of COX2 in fixed cryosections of nasopharynx in uninfected mice (control) or mice infected for five days with influenza A virus. Scale bars, 100 μm. Bottom, quantification of COX2 immunofluorescence in the nasopharynx of indicated mice. Data are mean ± s.e.m.; n = 29 sections from 5 mice per group over 3 independent experiments. Two-tailed unpaired t-test, P < 0.0001. Left panel in part a adapted with permission from ref. 35, Elsevier. Top panel in part b created with BioRender.com.
Centrally, sensory neurons of the nodose and petrosal ganglia cross the skull and innervate brainstem regions that include the nucleus of the solitary tract (NTS) and area postrema32, whereas jugular sensory neurons innervate the paratrigeminal nucleus33. Various Cre-defined vagal afferents target spatially restricted subregions of the NTS, with some but not all afferent types also accessing the area postrema24,34,35. The axons of NJP GABRA1 neurons were highly restricted in the lateral NTS (Fig. 5a), not observed in the area postrema, and remote from NTS locations targeted by axons of some other Cre-defined NJP neurons, such as gut-innervating neurons marked by expression of Gpr65 or Glp1r35. These findings further support a model for topographic organization in the brainstem36, with neurons relaying the presence of an airway infection spatially restricted from at least some other interoceptive inputs.
In the periphery, labelled GABRA1 axons were observed in only a few internal organs known to receive vagal and glossopharyngeal innervation. We observed densest innervation in the nasopharynx and oral cavity including taste papillae, some innervation of tracheal and pharyngeal muscles, and sparse innervation of stomach muscle (Extended Data Fig. 10b). Innervation was not observed in many other internal organs, including the heart, oesophagus, aorta and carotid sinus. The nasopharynx is a rich site for immune surveillance, as it connects the nasal cavity to the rest of the respiratory system and provides an early line of mucosal immune defence37. Influenza infection increases PGE2 levels locally within the nasopharynx38, and in humans leads to inflammation of nasopharyngeal tonsils39. Mice have an orthologous system known as nasopharynx-associated lymphoid tissue40, so we tested whether GABRA1 axons were located near relevant immune mediators. GABRA1 axons were enriched dorsally in epithelial and subepithelial layers of the nasopharynx (Fig. 5b) and notably, cyclooxygenase-2 expression was likewise detected in dorsal epithelium of the nasopharynx (Fig. 5c). Moreover, cyclooxygenase-2 expression was markedly upregulated in dorsal nasopharynx after influenza infection in mice (Fig. 5c). Thus, GABRA1 neurons occupy a strategic and privileged position for detection of the increased PGE2 levels that occur during viral infection.
Discussion
Despite seasonal vaccination and antiviral therapeutics, influenza A viral infection remains one of the most severe human threats, affecting millions of people worldwide each year. Cyclooxygenase-2 inhibitors are among the most prevalent anti-sickness and anti-pain medications, with around 30 billion doses of nonsteroidal anti-inflammatory drugs (NSAIDs) consumed annually in the USA alone41. Blockade of PGE2 production alleviates sickness, and several models have been proposed for how the brain receives input about a peripheral infection through PGE2 (ref. 2). Here, we observe that influenza-induced sickness behaviours were similarly attenuated by 1) ibuprofen, aspirin and EP3 receptor antagonism, 2) Ptger3-targeted gene knockout using Advillin-creER, Phox2b-cre and Gabra1-IRES-cre mice, and direct AAV-cre injection in NJP ganglia, 3) ablation of GABRA1 NJP neurons, and 4) and glossopharyngeal nerve transection. From these combined results, we conclude that a small cluster of Gabra1-expressing glossopharyngeal sensory neurons detects PGE2 and induces a neuronally orchestrated state of sickness associated with a suite of behavioural responses to respiratory viral infection.
Prostaglandins and many other cytokines can activate vagal and other peripheral afferents in vitro8,29,31, but their physiological roles in naturalistic infections have been difficult to parse. Vagotomy blocks sickness responses to cytokines and lipopolysaccharide in some studies but not others42,43, and this variability may be owing to the location or dose of pyrogen administration. Our findings indicate a clear role for sparse glossopharyngeal sensory neurons that are anatomically poised to detect influenza-induced PGE2. PGE2 has limited stability in vivo44, so peripheral sensory neurons that directly detect PGE2 at the infection site would seemingly provide a fast and robust conduit for information transfer to the brain.
Sickness behaviours have been proposed to help conserve energy to fight off infection1. However, eliminating the influenza detection pathway of the glossopharyngeal nerve not only attenuates sickness behaviour, but also promotes survival. Knockout of EP3 from a small group of glossopharyngeal neurons is sufficient to mimic the profound survival phenotype observed in full-body knockouts. One model is that pathogen-induced anorexia is beneficial in some infection models but harmful in others. For example, blocking PGE2 production is harmful during mycobacterium 3 infection45, but promotes survival during influenza infection19,46. Moreover, glucose gavage is detrimental during bacterial sepsis, where blood glucose may provide direct fuel for the pathogen, but protective during influenza infection47. Thus, disrupting neuronal responses to infection may decrease mortality by attenuating changes in feeding behaviour. Moreover, an infection-activated, anorexia-promoting neural pathway may provide a net survival benefit during certain bacterial infections, and thus is maintained over evolution, but is harmful during viral infection, as observed here. In addition, sickness behaviour may provide a separate population-level benefit as sick animals seek isolation and thereby limit pathogen transmission to kin48.
These findings indicate that targeted EP3 receptor knockout in sensory neurons not only affects sickness behaviour, but also the immune response and viral transition from the upper to lower respiratory tract. A parsimonious interpretation of these findings is that petrosal GABRA1 neurons, on detection of PGE2, engage neural circuits that evoke coordinated responses that include sickness behaviours as well as motor reflexes that affect immune function. Additionally, changes in feeding behaviour may secondarily affect immune function47,48 and activation of petrosal GABRA1 neurons may affect levels of cytokines other than PGE2 (Extended Data Fig. 9c), which can potentially elicit further neuronal feedback and enhance sickness behaviour. All such responses to infection would critically depend on the EP3 receptor in petrosal GABRA1 neurons, which serves an essential role in first relaying the presence of an upper respiratory infection to the brain, ultimately evoking sickness behaviour.
Influenza infection-induced sickness behaviour was attenuated, but not eliminated, following NSAID treatment, targeted EP3 receptor knockout, targeted neuronal ablation and glossopharyngeal nerve transection, suggesting other routes to sickness. Of note, the kinetics of residual sickness behaviour following manipulation of petrosal GABRA1 neurons matches the time course of virus accumulation in the lungs, indicating that there are probably two phases of influenza-induced sickness behaviour. The first phase occurs when the virus is most prevalent in the upper respiratory tract, and sickness is primarily mediated by PGE2-detecting glossopharyngeal sensory neurons marked by GABRA1, which project to the nasopharynx. The second phase of sickness occurs when the virus is most prevalent in the lower respiratory tract, and the lung is primarily innervated by vagal rather than glossopharyngeal sensory neurons. Our data raise the possibility that this second phase of sickness involves another neuronal pathway independent of PGE2, EP3 and glossopharyngeal sensory neurons. Inhibitors of residual neuro–immune interaction pathways, once identified, could potentially provide improved relief of influenza-induced malaise when used in combination with NSAIDs. We also note that PGE2 receptors are expressed in multiple NJP sensory neuron types, as well as in the brain and dorsal root ganglia. It is likely that the nervous system uses multiple sensory pathways to detect peripheral infections, with different sensors perhaps specialized for particular pathogen types or infection locations in the body. In this way, gut and respiratory sensory neurons could potentially engage different neural circuits that lead, for example, to either cough or nausea31,49. Consistent with this notion, various routes to sickness are differentially sensitive to pharmacological inhibition; for example, gut malaise is treated clinically using antagonists of the serotonin receptor HTR3A50. Understanding the diversity of sensory pathways to sickness and when they are engaged by different pathogen infections will provide an essential framework for deciphering this complex and poorly understood physiological state, and may enable improved therapeutic interventions.
Methods
Animals
All animal procedures followed the ethical guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the institutional animal care and use committee (IACUC) at Harvard Medical School. Mice were maintained under constant temperature (23 ± 1 °C) and relative humidity (46 ± 5%) with a 12-h light:dark cycle. Wild-type C57BL/6J (000664), Nestin-cre (003771), Phox2b-cre (016223), Advillin-creER (032027), Trpv1-IRES-cre (017769), Pdyn-IRES-cre (027958), Piezo2-EGFP-IRES-cre (027719), Oxtr-IRES-cre (031303), Agrp-cre (012899), lsl-DTR (007900), lsl-tdTomato (Ai14, 007914) were purchased from Jackson Laboratories. Gabra1-IRES-cre, lsl-L10-GFP and Ptger3flox mice were previously generated14,22,51.
Influenza A virus inoculation
Influenza A/PR/8/34 (H1N1) was purchased from Charles River Avian Vaccine Services (10100374) with a EID50 of 1012 ml−1. Virus was diluted in sterile saline for the administered dosage, which was EID50 106 ml−1 unless otherwise indicated. Viral administration was adapted from previous studies52. Awake mice were manually restrained and influenza A virus was delivered through each nostril (25 μl total volume) slowly using a P200 pipette to prevent spillage or delivery to the mouth. After administration, the mice were held with nostrils upright for 10–15 s, and returned to their cage.
Behavioural monitoring
For analysis of influenza A virus infection-induced behavioural changes, six- to eight-week-old mice without sex bias were singly housed with free access to food and water. Baseline values were obtained by measuring daily food intake, daily water intake, body weight, and motility for three days before inoculation. After viral infection, all measurements were expressed as a percentage change from baseline values. Food and water intake were calculated daily by measuring the change in weight of remaining food and water in the cage. Animal movement was recorded (C922x Pro Stream Webcam, Logitech) in the home cage for 30 min daily and motility expressed as the total distance moved, as determined by the MTrack2 plug-in available in ImageJ (1.53q, NIH). For measurement of acute food intake, mice were fasted for 24 h, and at dark onset, were administered with PGE2 or sulprostone (EP3 agonist, 14765, Cayman Chemical Company) via the exposure route and doses indicated in Figure legends. Animals were then singly housed in clean cages with ad libitum access to food and water, and the amount of food consumed for 1 h determined by measuring the food in the cage before and after the test period.
Body temperature measurements
Core body temperature was monitored by a rectal probe or radio telemetry. Rectal probe measurements were made hourly each day from noon to midnight using a mouse rectal temperature probe (Kent Scientific, RET-3, 0.16 mm tip diameter) connected to a thermometer (Kent Scientific WD-20250-9). Probes were sterilized (70% ethanol), lubricated (Aquagel lubricating gel, ParkerLabs, 57-05), and inserted ~5–7 mm into the rectum of physically restrained mice, and temperature was recorded for 30 s. For radio telemetry, a radio telemetry device that reports body temperature (HD-X11, DSI) was surgically implanted in the abdominal cavity. Surgery was performed under anaesthesia with avertin (200 mg kg−1, intraperitoneal injection) and the abdominal area was disinfected and shaved. A ~1 cm incision was made, and the abdominal peritoneum at the linea alba was gently exposed to insert the sterile radio telemetry device. The surgery site was sealed with VICRYL Sutures (J392H, Ethicon). Animals received Buprenorphine SR (BupSR-LAB, ZooPharm, 20 mg kg−1 subcutaneously at the back of the neck), and were allowed to recover for at least a week. Body temperature data were collected over 15 min intervals with a receiver (easyTEL, Emka Technologies) connected to a computer running iox software v.2.10.8. All data points from one mouse on a given day were averaged.
Pharmacological manipulations
Ibuprofen (I4883) and aspirin (acetylsalicylic acid, A5376) were purchased from Sigma, and PGE2 receptor antagonists SC-51322 (2791), PF-04418948 (4818), DG-041 (6240), and ONO-AE3-208 (3565) were purchased from Tocris. Ibuprofen (1 mg ml−1, saline) was added to drinking water ad libitum, and aspirin (20 mg kg−1, saline) and PGE2 receptor antagonists (1 mg kg−1, saline) were injected (intraperitoneally) daily. Administration of cyclooxygenase-2 inhibitors and PGE2 receptor antagonists began 3 days before virus inoculation and continued to the end of the monitoring period. Tamoxifen was administed daily for 5 days (intraperitoneal injection, 70 mg kg−1) at least one week before influenza virus infection.
Ganglion injection
NJP ganglia were injected with AAVs or diphtheria toxin as previously described34, with minor modifications. Mice were anaesthetized (200 mg kg−1 avertin, intraperitoneal injection), and depth of anaesthesia ensured throughout the procedure by lack of a toe pinch response. NJP ganglia were exposed and serially injected (10 × 13.8 nl) with saline solution containing either AAVs (titre > 6.7 × 1012 vg ml−1) or diphtheria toxin (5 μg ml−1, Sigma) supplemented with 0.05% Fast Green FCF Dye (Sigma) using a sharply pulled glass pipette attached to a Nanoject Injector (Drummond). AAV-cre (AAV-Syn-Cre-GFP, SignaGen Laboratories, SL100892, AAV9), AAV-flex-tdTomato (AAV9.CAG.Flex.tdTomato.WPRE.BGH, Addgene, 51503, AAV9), AAV-GFP (pENN.AAV.CB7.CI.eGFP.WPRE.rBG, Addgene, 105542, AAV9), and AAV-flex-AP (AAV9.CAG.flex.PLAP.WPRE.bgH, custom virus, Boston Children’s Hospital Viral Core, Boston, MA, AAV9) were purchased. Successful injection was verified by Fast Green FCF Dye slowly filling the entire ganglion without leakage. Surgical wounds were closed with coated VICRYL Sutures (J392H, Ethicon) and animals received Buprenorphine SR (BupSR-LAB, ZooPharm, 20 mg kg−1 subcutaneously at the back of the neck) as an analgesic. Animals recovered for at least two weeks for behavioural analysis or four weeks for histological analysis.
Nerve transection
Mice were anaesthetized (200 mg kg−1 avertin, intraperitoneal injection), and depth of anaesthesia ensured throughout the procedure by lack of a toe pinch response. NJP ganglia were surgically exposed, and the glossopharyngeal nerve was identified and severed immediately adjacent to the ganglia using microscissors. Sham surgeries involved NJP ganglia exposure without nerve transection. Surgical wounds were closed with coated VICRYL Sutures (J392H, Ethicon) and animals received 20 mg kg−1 Buprenorphine SR (BupSR-LAB, ZooPharm, subcutaneously at the back of the neck) as an analgesic. Animals recovered for at least two weeks before behavioural analysis.
Histological and anatomical analysis
Immunohistochemistry and analysis of native tissue fluorescence was performed after intracardial perfusion of fixative (10 ml PBS, then 10 ml 4% paraformaldehyde in PBS). For whole-mount DTR immunostaining, NJP ganglia were collected and permeabilized (11.5 g glycine, 400 ml PBS with 0.2% Triton X-100, 100 ml DMSO) at 37 °C for 1 week. Ganglia were then blocked (1 h, room temperature) in blocking buffer (5% normal donkey serum, 017-000-121, Jackson in 0.05% Tween-20/PBS) and incubated (overnight, 4 °C) with 1:200 anti-DTR (HB-EGF, Goat, AF259NA, Fisher Scientific) in blocking buffer. Samples were washed (4 × 10 min, 0.05% Tween-20 in PBS, room temperature) and incubated (2 h, room temperature) with anti-goat Alexa 488 solution (Jackson Immunoresearch, 705-545-147, 1:500 in PBS with 0.05% Tween-20). Tissues were washed again (4 × 10 min, 0.05% Tween-20 in PBS, room temperature), mounted (Fluoromount G medium, SouthernBiotech) onto microscope slides (Fisher Scientific), and visualized using a Leica SP5 II confocal microscope and analysed using ImageJ (1.53q). For cyclooxygenase-2 immunostaining, nasopharyngeal tissue was cryosectioned (15 μm), blocked (1 h, room temperature) with blocking solution (5% normal donkey serum, PBS with 0.01% Triton X-100), and incubated (overnight, 4 °C) with anti-COX2 antibody (rabbit, ab179800, Abcam, 1:500 in PBS with 0.01% Triton X-100). Sections were washed (3 × 10 min, room temperature, PBS with 0.01% Triton X-100) and incubated (2 h, room temperature) with anti-rabbit Alexa 488 solution (Jackson Immunoresearch, 711-545-152, 1:1,000 in PBS with 0.01% Triton X-100). Sections were washed again (3 × 10 min, room temperature, PBS with 0.01% Triton X-100), mounted with Fluoromount G medium, and visualized using a Leica SP5 II confocal microscope. To visualize brainstem axons, brainstem cryosections (20 μm) were obtained from AAV-injected mice, and processed as described above for cyclooxygenase-2 immunostaining in nasopharyngeal tissue, except antibodies were 1:1,000 anti-GFP (chicken, GFP-1020, Aves Labs), 1:1,000 anti-RFP (rabbit, 600-401-379, Rockland), anti-chicken Alexa 647 (Jackson Immunoresearch, 703-605-155, 1:300) and anti-rabbit Cy3 (Jackson Immunoresearch, 111-165-144, 1:300). To visualize axons in nasopharynx, fixed nasopharyngeal (15 μm) sections were stained as above for cyclooxygenase-2 immunostaining, but with anti-RFP primary antibody followed by anti-rabbit Cy3 secondary antibody. For GABRA1 neuron innervation to the trachealis and inferior pharyngeal constrictor muscles, tissues were collected fresh, cut along the ventral axis for open-book visualization, fixed (4% paraformaldehyde/PBS either 1 h, room temperature or overnight, 4 °C), and stained for tdTomato as above for tdTomato visualization in nasopharynx, except primary antibody incubation (36 h, 4 °C) and subsequent washes (3 × 12 h, 4 °C) were longer. For visualizing alkaline phosphatase, animals were perfused with PBS, and tissues were collected, fixed (4% paraformaldehyde, 1 h, room temperature), and washed in cold PBS. Tissues were then incubated (70 °C, 2 h) in alkaline phosphatase buffer (0.1 M Tris HCl pH 9.5, 0.1 M NaCl, 50 mM MgCl2, 0.1% Tween-20, 5 mM levamisole) and washed twice in alkaline phosphatase buffer. Alkaline phosphatase activity was visualized with NCT/BCIP solution (34042, ThermoFisher Scientific) according to manufacturer’s protocols. Stained samples were post-fixed (4% paraformaldehyde, overnight, 4 °C), dehydrated through a series of ethanol washes, and cleared using a 1:2 mixture of benzyl alcohol (Sigma-Aldrich 402834-500ML): benzyl benzoate (Sigma-Aldrich B6630-1L). The tissue was then cut along the ventral axis for open-book visualization. Whole-mount stainings were captured by microscopy with an AxioZoom (Zeiss) and analysed using ImageJ (1.53q).
Analysis of mRNA expression in situ
DNA-based hybridization chain reaction (HCR) probes against Ptger3 (lot no. PRH698) and Phox2b (lot no. PRF341) were purchased from Molecular Instruments. Hybridization solution (30% formamide, 5× SSC, 9 mM citric acid (pH 6), 0.1% Tween-20, 50 μg ml−1 heparin, 1× Denhardt’s solution, 10% dextran sulfate), probe wash buffer (30% formamide, 5× SSC, 9 mM citric acid pH 6.0, 0.1% Tween-20, 50 μg ml−1 heparin), amplification buffer (5× SSC, 0.1% Tween-20, 10% dextran sulfate), and fluorophore-labelled HCR amplifiers were purchased from Molecular Instruments. NJP ganglia were freshly collected, mounted in OCT (Sakura Finetek) and cryosectioned (10 μm). Tissue was post-fixed (4% PFA, PBS, room temperature, 20 min), washed (3 × 10 min, PBS, room temperature), treated with 1% hydrogen peroxide (PBS, room temperature, 20 min), and incubated with Ptger3 and Phox2b HCR probes (0.4 pmol, hybridization buffer in a humidified chamber, 37 °C, overnight). Tissue was then washed (15 min, 37 °C) with (1) 3:1 probe wash buffer: 5× SSCT (5× SSC, 0.1% Tween-20), (2) 1:1 probe wash buffer: 5× SSCT, (3) 1:3 probe wash buffer: 5× SSCT, and (4) 5× SSCT. Tissue was then incubated with amplification buffer (30 min, room temperature), and then with fluorescent HCR amplifiers (6 pmol, Alexa594 for Ptger3 probe and Alexa 488 for Phox2b probe) in amplification buffer (humidified chamber, room temperature, overnight). Tissue was then washed (5× SSCT, 2 × 30 min, 1 × 5 min, room temperature), mounted in Fluoromount G medium, and visualized with by confocal microscopy (Leica SP5 II).
Single-cell transcriptomics
All UMAP plots in this manuscript were made from published single-cell transcriptome data (GEO Accession ID: GSE145216)22 using Seurat (4.1.0) and R Studio (4.1.2).
Quantitative PCR analysis
Levels of mouse Ptger3, Gapdh transcript and the influenza virus nucleoprotein (NP) were measured in cDNA obtained from hypothalamus, NJP ganglia, nasal lavage (for upper respiratory tract analysis), and/or BALF (for lower respiratory tract analysis). BALF was collected by surgically opening the neck and cannulating a 21G catheter into the trachea. In total, 3 × 1 ml of PBS was slowly injected into the lungs then solution was gently aspirated back to the syringe and collected. Collected BALF was centrifuged (7 min, 400g, 4 °C), and the supernatants were stored (−80 °C) until analysis. Nasal lavage was performed by inserting a needle (26G) into the upper trachea angled toward the nose. Sterile PBS (1 ml) was administered through the needle via a cannulated syringe (PE20 polyethylene cannula, inner diameter 0.38 mm), and collected after expulsion from the nose. RNA was collected and cDNA synthesized from BALF, nasal lavage fluid, and homogenized tissue using Trizol (15596026, ThermoFisher) and the High-Capacity cDNA Reverse Transcription Kit (4368813, ThermoFisher) according to the manufacturer’s protocols. qPCR analysis was performed on cDNA using gene-specific primers, PowerTrack SYBR Green Master Mix (A46012, ThermoFisher) on QuantStudio 7 qPCR instrument (ThermoFisher). Gapdh primers were GGGTGTGAACCACGAGAAATATG and TGTGAGGGAGATGCTCAGTGTTG; Ptger3 primers were CAACCTTTTCTTCGCCTCTG and TTTCTGCTTCTCCGTGTGTG; and influenza virus nucleoprotein primers were GACGATGCAACGGCTGGTCTG and ACCATTGTTCCAACTCCTTT. Expression levels of Ptger3 and nucleoprotein were normalized to Gapdh levels. Normalization was by the 2–ΔCT method for Extended Data Fig. 4a, and the 2–ΔΔCT method53 for Extended Data Fig. 9b, with added normalization using values from uninfected controls.
PGE2 and cytokine measurements
Mice were euthanized at different time points after infection with influenza A virus. Whole blood was collected by cardiac puncture using a 0.5 M EDTA-coated needle and syringe and mixed immediately with 0.5 M EDTA (50 μl). Plasma was isolated by centrifugation (3,000 rpm, 10 min, 4 °C) and the supernatant stored (−80 °C) until ELISA analysis. BALF was collected as described above. PGE2 and cytokine levels were measured in plasma and/or BALF by ELISA (R&D Systems, PGE2: KGE004B, TNF: DY410-5, IFNγ: DY485-05, IL-6: DY406-05) according to the manufacturer’s instructions.
Calcium imaging
NJP ganglia were acutely collected from Gabra1-IRES-cre; lsl-tdTomato mice and incubated (37 °C, 90 min, with rotation) in dissociation solution (Dulbecco’s Modified Eagle’s Medium (DMEM) containing liberase (55 mg ml−1, Roche, 05401135001), DNase (0.004%, Worthington, LS002007)). Cells were than pelleted (3 min, 300g, 4 °C) and resuspended in 0.2% ovomucoid, 0.004% DNase, DMEM, 200 μl. Using a P200 pipette, cells were triturated at least 10 times until clumps were not visible, filtered through a 40 μm mesh cell strainer, and pelleted again (3 min, 300g, 4 °C). Cells were then resuspended in culture medium containing 5 μM Calbryte 520 AM (20651, AAT Bioquest); culture medium contains 1× B27 (Gibco, 17504044), 1× N2 (Gibco, 17502048), 1× penicillin and streptomycin (Gibco, 15140122) in Neurobasal media without phenol red (Gibco, 12348017). Resuspended cells were then plated onto laminin-coated cover glass and incubated (37 °C, at least 30 min before imaging) in a humidified CO2 incubator. The cover glass was then transferred to a chamber with an inlet and outlet perfusion (Warner Instruments, RC-24N) with continuous flow of Hank’s balanced salt solution (HBSS). The calcium responses were then imaged (488 nm) on perfusion of prostaglandin E2 (1 μM in HBSS, 2 min) and KCl (150 mM, at the end of the run). GABRA1-positive neurons were identified by tdTomato fluorescence (568 nm). Cells were identified as responsive if mean Calbryte 520 AM fluorescence during the stimulation period exceeded three standard deviations above the baseline mean.
Fibre photometry
Fibre photometry of AGRP neurons was performed as described54 with PGE2 (intraperitoneal injection, 0.5 mg kg−1) or PBS acutely injected during a brief manual restraint using Synapses software (Build: 94-42329P, Tucker-Davis Technology). Data were analysed using Matlab (R2020a).
Statistical analysis
Data in graphs are represented as mean ± s.e.m., with all sample sizes and P values provided in figure legends. Statistical analysis for behavioural experiments involved averaging daily changes in parameters indicated per mouse (days 1–10 after infection or through survival). Statistical significance was calculated with Prism 8.4.3 software (GraphPad), and involved statistical tests described in figure legends. Probit analysis in Extended Data Fig. 5b was performed with SPSS, 21 (IBM). Sample sizes were chosen based on prior expertise and publications in our field26,55 and are disclosed in the Figure legends. Animal groups were randomly assigned and control animals were age-matched to experimental animals. The same investigator performed genotyping and analysis of sickness responses, so data were not generated blind to genotype or experimental group.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-023-05796-0.
Supplementary information
Acknowledgements
We thank C. Saper for Ptger3flox mice and comments on the manuscript. Cartoons were created using BioRender.com. This work was supported by grants from the NIH (DP1 AT009497 and R01 HL132255) and the Chan Zuckerberg Initiative to S.D.L., a Banting Postdoctoral Fellowship to N.-R.B. and a Harvard Medical School Goldberg Fellowship to N.H. S.L.P. was a Warren Alperts Distinguished Scholar and S.D.L. is an investigator of the Howard Hughes Medical Institute.
Extended data figures and tables
Source data
Author contributions
N.-R.B., S.L.P., N.H., I.M.C. and S.D.L. designed experiments. N.-R.B., N.H., S.L.P. and Y.W. performed experiments. N.-R.B., S.L.P., N.H., I.M.C. and S.D.L. analysed data. N.-R.B. and S.D.L. wrote the manuscript.
Peer review
Peer review information
Nature thanks Alice McGovern, Ruslan Medzhitov and Kevin Tracey for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All data used to generate figures, including behavioural data points from each individual mouse are provided as source data. All reagents that may not be commercially available including certain transgenic mice and AAVs will be made freely available upon reasonable request. Source data are provided with this paper.
Competing interests
S.D.L. is a consultant for Kallyope, Inc. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41586-023-05796-0.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-023-05796-0.
References
- 1.Poon DC, Ho YS, Chiu K, Wong HL, Chang RC. Sickness: from the focus on cytokines, prostaglandins, and complement factors to the perspectives of neurons. Neurosci. Biobehav. Rev. 2015;57:30–45. doi: 10.1016/j.neubiorev.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinho-Ribeiro FA, Verri WA, Jr., Chiu IM. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol. 2017;38:5–19. doi: 10.1016/j.it.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen L, et al. The impact of influenza epidemics on mortality: introducing a severity index. Am. J. Public Health. 1997;87:1944–1950. doi: 10.2105/AJPH.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart BL. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 8.Zanos TP, et al. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc. Natl Acad. Sci. USA. 2018;115:E4843–E4852. doi: 10.1073/pnas.1719083115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu IM, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy MK, Weinberg JB. Eicosanoids and respiratory viral infection: coordinators of inflammation and potential therapeutic targets. Mediators Inflamm. 2012;2012:236345. doi: 10.1155/2012/236345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec JD. Prostaglandins and sickness behavior: old story, new insights. Physiol. Behav. 2009;97:279–292. doi: 10.1016/j.physbeh.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 13.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus M, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 15.Dantzer R, et al. Molecular basis of sickness behavior. Ann. NY Acad. Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang TA, et al. Thermoregulation via temperature-dependent PGD2 production in mouse preoptic area. Neuron. 2019;103:309–322.e7. doi: 10.1016/j.neuron.2019.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterhout JA, et al. A preoptic neuronal population controls fever and appetite during sickness. Nature. 2022;606:937–944. doi: 10.1038/s41586-022-04793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhaveri KA, Trammell RA, Toth LA. Effect of environmental temperature on sleep, locomotor activity, core body temperature and immune responses of C57BL/6J mice. Brain Behav. Immun. 2007;21:975–987. doi: 10.1016/j.bbi.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulombe F, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Lau J, et al. Temporal control of gene deletion in sensory ganglia using a tamoxifen-inducible Advillin-Cre-ERT2 recombinase mouse. Mol. Pain. 2011;7:100. doi: 10.1186/1744-8069-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sisley S, et al. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Invest. 2014;124:2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prescott SL, Umans BD, Williams EK, Brust RD, Liberles SD. An airway protection program revealed by sweeping genetic control of vagal afferents. Cell. 2020;181:574–589.e14. doi: 10.1016/j.cell.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescott SL, Liberles SD. Internal senses of the vagus nerve. Neuron. 2022;110:579–599. doi: 10.1016/j.neuron.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai L, et al. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179:1129–1143.e23. doi: 10.1016/j.cell.2019.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kupari J, Haring M, Agirre E, Castelo-Branco G, Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019;27:2508–2523.e4. doi: 10.1016/j.celrep.2019.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baral P, et al. Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 2018;24:417–426. doi: 10.1038/nm.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min S, et al. Arterial baroreceptors sense blood pressure through decorated aortic claws. Cell Rep. 2019;29:2192–2201.e3. doi: 10.1016/j.celrep.2019.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baba H, Kohno T, Moore KA, Woolf CJ. Direct activation of rat spinal dorsal horn neurons by prostaglandin E2. J. Neurosci. 2001;21:1750–1756. doi: 10.1523/JNEUROSCI.21-05-01750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleridge HM, et al. Stimulation of ‘irritant’ receptors and afferent C-fibres in the lungs by prostaglandins. Nature. 1976;264:451–453. doi: 10.1038/264451a0. [DOI] [PubMed] [Google Scholar]
- 30.Verzele NAJ, et al. The impact of influenza pulmonary infection and inflammation on vagal bronchopulmonary sensory neurons. FASEB J. 2021;35:e21320. doi: 10.1096/fj.202001509R. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed SP, Higenbottam TW, Adcock JJ. Effects of aerosol-applied capsaicin, histamine and prostaglandin E2 on airway sensory receptors of anaesthetized cats. J. Physiol. 1993;469:51–66. doi: 10.1113/jphysiol.1993.sp019804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 33.McGovern AE, et al. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J. Neurosci. 2015;35:7041–7055. doi: 10.1523/JNEUROSCI.5128-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal sensory neuron subtypes that differentially control breathing. Cell. 2015;161:622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams EK, et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ran C, Boettcher JC, Kaye JA, Gallori CE, Liberles SD. A brainstem map for visceral sensations. Nature. 2022;609:320–326. doi: 10.1038/s41586-022-05139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tacchi L, et al. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat. Commun. 2014;5:5205. doi: 10.1038/ncomms6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam VC, et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154:213–227. doi: 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005;5:718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green GA. Understanding NSAIDs: from aspirin to COX-2. Clin. Cornerstone. 2001;3:50–60. doi: 10.1016/S1098-3597(01)90069-9. [DOI] [PubMed] [Google Scholar]
- 42.Konsman JP, Luheshi GN, Bluthe RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci. 2000;12:4434–4446. doi: 10.1046/j.0953-816X.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- 43.Sehic E, Blatteis CM. Blockade of lipopolysaccharide-induced fever by subdiaphragmatic vagotomy in guinea pigs. Brain Res. 1996;726:160–166. doi: 10.1016/0006-8993(96)00326-5. [DOI] [PubMed] [Google Scholar]
- 44.Forstermann U, Neufang B. Elimination from the circulation of cats of 6-keto-prostaglandin E1 compared with prostaglandins E2 and I2. J. Pharm. Pharmacol. 1983;35:724–728. doi: 10.1111/j.2042-7158.1983.tb02878.x. [DOI] [PubMed] [Google Scholar]
- 45.Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat. Immunol. 2010;11:751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carey MA, et al. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J. Immunol. 2005;175:6878–6884. doi: 10.4049/jimmunol.175.10.6878. [DOI] [PubMed] [Google Scholar]
- 47.Wang A, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166:1512–1525.e12. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao S, et al. Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell. 2017;168:503–516.e12. doi: 10.1016/j.cell.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, et al. Area postrema cell types that mediate nausea-associated behaviors. Neuron. 2021;109:461–472.e5. doi: 10.1016/j.neuron.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freeman AJ, Cunningham KT, Tyers MB. Selectivity of 5-HT3 receptor antagonists and anti-emetic mechanisms of action. Anticancer Drugs. 1992;3:79–85. doi: 10.1097/00001813-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Krashes MJ, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanson, L. R., Fine, J. M., Svitak, A. L. & Faltesek, K. A. Intranasal administration of CNS therapeutics to awake mice. J. Vis. Exp.10.3791/4440 (2013). [DOI] [PMC free article] [PubMed]
- 53.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 54.Horio N, Liberles SD. Hunger enhances food-odour attraction through a neuropeptide Y spotlight. Nature. 2021;592:262–266. doi: 10.1038/s41586-021-03299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ilanges A, et al. Brainstem ADCYAP1+ neurons control multiple aspects of sickness behaviour. Nature. 2022;609:761–771. doi: 10.1038/s41586-022-05161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonomura K, et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541:176–181. doi: 10.1038/nature20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used to generate figures, including behavioural data points from each individual mouse are provided as source data. All reagents that may not be commercially available including certain transgenic mice and AAVs will be made freely available upon reasonable request. Source data are provided with this paper.