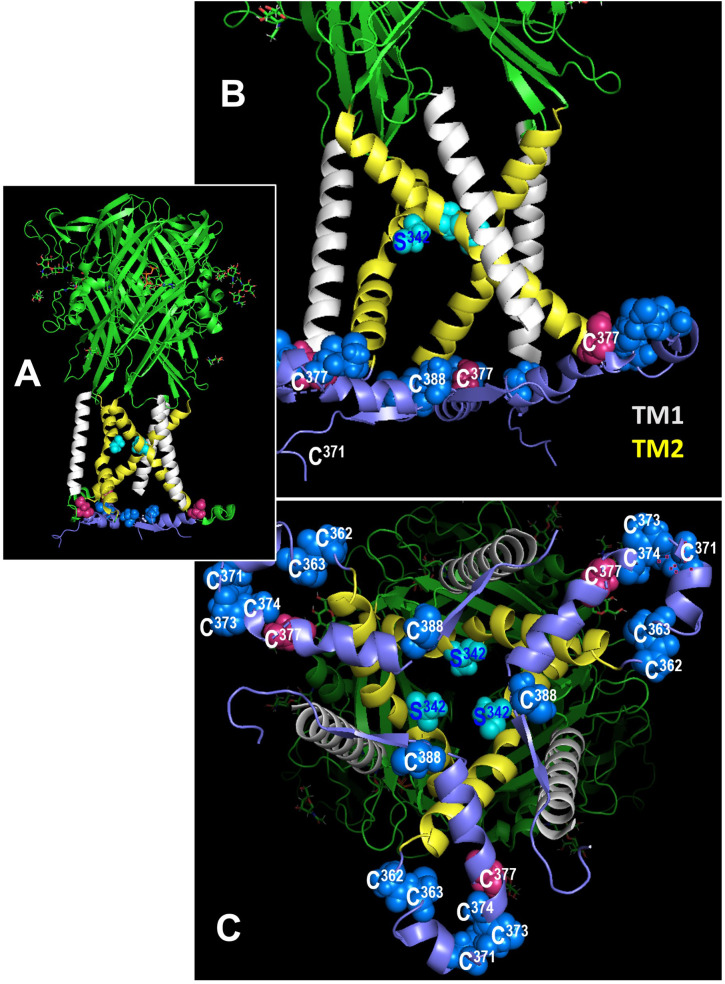
Figure 8.
Location of cysteines C377 and C388 in the rat P2X7 receptor (rP2X7R) in the open channel conformation. In all figures, the C-terminal residues 400-595 to the end of the 595 residues long subunits are hidden for simplicity. (A) Side view of the large extracellular domain harboring the ATP binding sites, the six transmembrane domains (3 x TM1 in white, 3 x TM2, in yellow), and an interfacial helically structured domain lying parallel to the membrane plane. Also shown are the three S342 residues, which constitute the channel gate (46). (B) Enlarged side view of the transmembrane domains, the S342 residues (cyan) and the position of two (of three each) residues C377 and C388 in the interfacial region. The other blue spheres indicate additional cysteines of the cysteine-rich domain. (C) Perpendicular view from the cytoplasmic side on the numerous cysteine residues of the interfacial region and the transmembrane domains above.