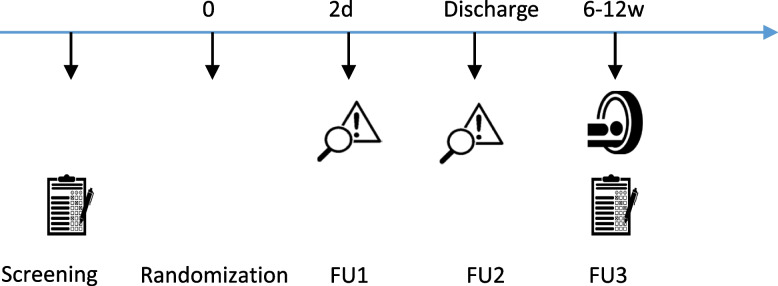
Fig. 2.
Flowchart of “L-PRF in cranial surgery, a prospective randomized controlled trial.” After obtaining informed consent, patients are screened and asked to complete quality of life (QoL) questionnaires (EQ-5D and RAND SF-36). During surgery (i.e., before dural closure), study subjects are randomized into the experimental arm (L-PRF) and the control arm (commercially available fibrin sealants). During hospitalization, patients are followed up with clinical controls after 2 days (FU1) and on the day of discharge (FU2). One outpatient follow-up is done between 6 and 12 weeks postop (FU3), with MRI imaging and completion of the QoL questionnaires