Abstract
Background:
Radiation necrosis (RN) is a clinically relevant complication of stereotactic radiosurgery (SRS) for intracranial metastasis (ICM) treatments. Radiation necrosis development is variable following SRS. It remains unclear if risk factors for and clinical outcomes following RN may be different for melanoma patients. We reviewed patients with ICM from metastatic melanoma to understand the potential impact of RN in this patient population.
Methods:
Patients who received SRS for ICM from melanoma at Mayo Clinic Arizona between 2013 and 2018 were retrospectively reviewed. Data collected included demographics, tumor characteristics, radiation parameters, prior surgical and systemic treatments, and patient outcomes. Radiation necrosis was diagnosed by clinical evaluation including brain magnetic resonance imaging (MRI) and, in some cases, tissue evaluation.
Results:
Radiation necrosis was diagnosed in 7 (27%) of 26 patients at 1.6 to 38 months following initial SRS. Almost 92% of all patients received systemic therapy and 35% had surgical resection prior to SRS. Patients with RN trended toward having larger ICM and a prior history of surgical resection, although statistical significance was not reached. Among patients with resection, those who developed RN had a longer period between surgery and SRS start (mean 44 vs 33 days). Clinical improvement following treatment for RN was noted in 2 (29%) patients.
Conclusions:
Radiation necrosis is relatively common following SRS for treatment of ICM from metastatic melanoma and clinical outcomes are poor. Further studies aimed at mitigating RN development and identifying novel approaches for treatment are warranted.
Keywords: Radiation necrosis, brain metastasis, intracranial metastasis, melanoma, stereotactic radiosurgery
Introduction
Melanoma has a high propensity for intracranial metastases (ICMs), with most patients presenting with ICM at diagnosis. Advances in detection of and systemic therapy for melanoma have contributed to improved patient survival and subsequently an increased prevalence of patients with ICM. Both the presence and treatment-related complications of ICM contribute to neurocognitive complications that impact patient morbidity and mortality.1-6 Current treatment strategies for ICM from melanoma include systemic therapies, whole-brain radiation therapy (WBRT), surgical resection, and stereotactic radiosurgery (SRS) or radiotherapy.7-10 Stereotactic techniques are a desirable option in patients with manageable disease burden, as they are less invasive and costly than surgery, and more targeted than WBRT.8 Ongoing clinical trials will further clarify whether SRS’s advantage over WBRT exists also in patients with several brain metastases (>4).10 In melanoma in particular, SRS can overcome potentially tumor-inherent radioresistance compared with WBRT.11 Rarely, SRS can have immediate or delayed toxicity-related complications, including seizures, neurological deficits, and ataxia.8,12
Radiation necrosis is an important dose-limiting, long-term toxicity seen after SRS that can present anywhere from months to years following radiation.13-15 Incidence varies in the literature from 5% to 29%, likely impacted by differences in definition, follow-up, and diagnoses between studies.12,13,16-19 In practice, it remains difficult to distinguish RN from tumor progression (TP) on initial presentation. Tissue diagnosis of RN is the gold standard. However, in cases where lesion resection is not possible, indicated, or desired, diagnosis may be inferred with variable confidence from imaging and patient presentation over time.13,20-23
Given RN uniquely impacts treatment strategy, it is critical to understand the possible risk factors for developing RN to aid in accurate diagnosis, triage, and management.12-14,24 Multiple studies support higher SRS dosing and larger tumor size as risk factors for RN.25-27 Recently, tumor biology has emerged as an important element of risk stratification.14 We retrospectively reviewed our single-institutional experience to understand the risk and treatment experience of RN in patients who received SRS for metastatic melanoma.
Materials and Methods
Patient inclusion
With institutional review board approval, we conducted a retrospective review of adult patients (age 18 or greater) with melanoma who received SRS for ICM between 2013 and 2018 at Mayo Clinic Arizona. Patients who received SRS alone, as either definitive therapy or adjuvant treatment after surgical resection, or a combination of SRS and WBRT were included. Patients who received only WBRT were excluded. One patient who received radiation at an outside institution had records available in addition to follow-up magnetic resonance imaging (MRI) at our institution and was therefore included in the analysis.
Data collection
Detailed chart review through December 2020 was undertaken to record patient demographics, date of initial melanoma diagnosis, tumor characteristics, radiation parameters, and other treatment modalities including surgery and various types of systemic therapy. We also collected data on clinical and radiographic response to radiation, diagnosis of RN, management of RN, and outcomes of RN-directed treatment.
SRS delivery
For patients treated at Mayo Clinic Arizona, SRS was delivered using a Linac-based frameless technique with 6 MV photons and the Exactrac system for image guidance. A thermoplastic mask was used for immobilization. Computed tomography (CT) simulation was performed at 1 mm slice thickness through the entire cranial volume, typically with intravenous contrast. Computed tomography images were then fused to high-resolution treatment planning MRI images obtained at 1 mm slice thickness using an axial T1-weighted sequence. Multileaf collimation techniques used based on the size and shape of the lesion included circular cones, dynamic multileaf collimation, or volumetric modulated arc therapy. Dose selection was determined considering tumor size, location, resection status, history of prior cranial radiation, and the number of lesions that required treatment. It was typically prescribed to the 80% isodose line. Stereotactic radiosurgery doses ranged from 14 to 24 Gy delivered in a single fraction, and for stereotactic radiotherapy doses ranged from 20 to 35 Gy delivered over multiple fractions, typically 3 or 5. Whole-brain radiation therapy was given over 10 to 15 fractions, with doses ranging from 30 to 36 Gy. Data were collected to determine patients who met target dosimetric goals (V12 < 10 ccs, V14 < 7 ccs) as per previously validated studies.28,29 Patients usually underwent surveillance with repeat brain MRI at 4 to 12 weeks after radiation, and thereafter at regular intervals or if symptomatic as recommended by their providers in oncology disciplines.
Outcomes
We reviewed all relevant clinical notes from all cancer-treating physicians. We comprehensively evaluated the discovery of radiographic and/or pathologic diagnosis of RN with or without concurrent TP. Radiation necrosis was defined as radiographic changes interpreted by the neuroradiologist as increased lesion size and surrounding edema in a previously irradiated lesion. In one instance, advanced imaging with MRI perfusion demonstrated decreased cerebral blood volumes to increase confidence in the diagnosis. The treating neuro-oncologist then determined whether the patient was symptomatic for this radiographic finding to determine clinical recommendations. Pathologic confirmation of RN occurred in 4 out of 7 patients, or 57%. We also recorded management strategies for RN including steroids, surgery, laser ablation, bevacizumab, additional systemic therapy in the case of concurrent TP, and/or the decision not to deliver specific therapy. If RN-directed therapy was given, we noted the patient’s outcome as improvement, stability, or progression of RN.
Statistical methods
Demographic, lesion, and treatment data for patients with and without RN were compared using Wilcoxon rank-sum test or Fisher’s exact test when applicable. A Kaplan-Meier curve using the time-varying predictor was generated and log rank test was used to identify any significant difference in survival between patients with and without RN.30 P values < .05 were considered statistically significant. Analyses were performed in SAS version 9.4 (SAS Institute; Cary, NC).
Results
Patient demographics
Of 26 consecutive patients included for final review, 7 were diagnosed with RN (27%). Patient demographics were comparable between groups (Table 1). Although patients with RN were younger on average than patients without RN (51 vs 61 years), this difference was not statistically significant. Somatic alterations known to be associated with melanoma were common in this cohort (Table 1). No patients were positive for MEK1 mutation. Molecular mutation status did not vary significantly between patients with and without RN.
Table 1.
Patient characteristics.
| Characteristic | Any RN diagnosis (N = 7) | No RN diagnosis (N = 19) | Total (N = 26) | P value |
|---|---|---|---|---|
| Age | 0.19a | |||
| Mean (SD) | 51.3 (16.1) | 61.2 (15.1) | 58.5 (15.7) | |
| Median | 56.0 | 63.0 | 62.0 | |
| Range | (32.0-70.0) | (30.0-86.0) | (30.0-86.0) | |
| Sex | 1.00b | |||
| Male | 5 (71%) | 12 (63%) | 17 (65%) | |
| Female | 2 (29%) | 7 (37%) | 9 (35%) | |
| Race | 0.27b | |||
| White | 6 (86%) | 19 (100%) | 25 (96%) | |
| Other | 1 (14%) | 0 (0.0%) | 1 (3.8%) | |
| BRAF V600 | 0.66b | |||
| Negative | 4 (57.1%) | 13 (68.4%) | 17 (65.4%) | |
| Positive | 3 (42.9%) | 6 (31.6%) | 9 (34.6%) | |
| NRAS | 0.29b | |||
| Negative | 4 (57.1%) | 16 (84.2%) | 20 (76.9%) | |
| Positive | 3 (42.9%) | 3 (15.8%) | 6 (23.1%) |
Abbreviations: BRAF, v-raf murine sarcoma viral oncogene homolog B1; RN, radiation necrosis; NRAS, neuroblastoma ras viral oncogene homolog; SD, standard deviation.
Wilcoxon.
Fisher exact.
Lesion characteristics
Mean time between initial melanoma diagnosis and identification of ICM on brain MRI was 70 months (median, 28 months; range, 0-353 months). This period was longer in patients ultimately diagnosed with RN than patients who did not develop RN (mean, 89 months vs 63 months); however, this difference was not statistically significant. Characteristics of ICM are described in Table 2. Patients diagnosed with RN did have a higher average value for lesion size (1.7 vs 1.2 cm), and also maximum lesion size (2.0 vs 1.6 cm), compared with patients without RN, although these differences were not statistically significant. Lesion number, laterality, distribution, and location were not associated with the development of RN. The presence of additional baseline lesion features including edema, cysts, and hemorrhage did not differ significantly between patients with and without RN. However, most lesions (73%) had associated edema, and this trend was proportionately higher in the group that subsequently developed RN.
Table 2.
Characteristics of intracranial metastases in patients with and without radiation necrosis after stereotactic radiosurgery.
| ICM characteristic | Any RN diagnosis (N = 7) | No RN diagnosis (N = 19) | Total (N = 26) | P value |
|---|---|---|---|---|
| Number of lesions treated per patient | 0.22a | |||
| Mean (SD) | 1.7 (1.3) | 2.7 (2.4) | 2.4 (2.2) | |
| Median | 1.0 | 2.0 | 2.0 | |
| Range | (1.0-4.0) | (1.0-11.0) | (1.0-11.0) | |
| Average lesion size by maximum diameter (cm) | 0.15a | |||
| Mean (SD) | 1.7 (1.0) | 1.2 (1.0) | 1.3 (1.0) | |
| Median | 1.6 | 0.8 | 1.0 | |
| Range | (0.4-3.2) | (0.2-4.3) | (0.2-4.3) | |
| Maximum lesion size (cm) | 0.35a | |||
| Mean (SD) | 2.0 (1.2) | 1.6 (1.2) | 1.7 (1.2) | |
| Median | 1.6 | 1.3 | 1.4 | |
| Range | (0.6-3.6) | (0.2-4.3) | (0.2-4.3) | |
| ICM number of locations | 0.29a | |||
| Mean (SD) | 1.4 (0.8) | 1.9 (1.2) | 1.8 (1.1) | |
| Median | 1.0 | 2.0 | 1.0 | |
| Range | (1.0-3.0) | (1.0-5.0) | (1.0-5.0) | |
| Lesion location | ||||
| Frontal | 4 (57%) | 11 (58%) | 15 (58%) | 1.00b |
| Occipital | 1 (14%) | 3 (16%) | 4 (15%) | 1.00b |
| Parietal | 2 (29%) | 9 (47%) | 11 (42%) | 0.66b |
| Temporal | 2 (29%) | 4 (21%) | 6 (23%) | 1.00b |
| Cerebellar | 1 (14%) | 6 (32%) | 7 (27%) | 0.63b |
| Brainstem | 0 (0.0%) | 1 (5.3%) | 1 (3.8%) | 1.00b |
| Basal ganglia | 0 (0.0%) | 3 (16%) | 3 (12%) | 0.54b |
| Additional ICM features | ||||
| Hemorrhage | 3 (43%) | 4 (21%) | 7 (27%) | 0.34b |
| Cysts | 1 (14%) | 0 (0.0%) | 1 (3.8%) | 0.27b |
| Edema | 6 (86%) | 13 (68%) | 19 (73%) | 0.63b |
Abbreviations: cm, centimeters; ICM, intracranial metastases; RN, radiation necrosis; SD, standard deviation.
Wilcoxon.
Fisher exact.
ICM treatment characteristics
Characteristics of ICM treatment prior to the development of RN are described in Table 3. Nearly all patients (92%) received one or a combination of systemic therapies prior to radiation. Systemic therapy type and number of different treatments did not vary significantly between patients with and without RN. There was a trend toward higher cumulative exposure of either cytotoxic chemotherapy or targeted therapy prior to radiation therapy in patients who subsequently developed RN; however, no trend was observed for patients who received immune therapy prior to radiation. Less than half of patients (35%) underwent surgical resection prior to radiation. A larger percentage of patients who developed RN had undergone surgical resection compared with those who did not develop RN (57% vs 26%), although this association was not statistically significant. There was a trend toward development of RN following a higher average number of days elapsed between surgery and adjuvant SRS, with 44 days elapsed in patients who developed RN compared with 33 days in patients who did not develop RN.
Table 3.
Therapies administered and the development of radiation necrosis.
| Treatment characteristic | Any RN diagnosis (N = 7) | No RN diagnosis (N = 19) | Total (N = 26) | P value |
|---|---|---|---|---|
| Prior radiation therapy | ||||
| WBRT | 0 (0.0%) | 2 (10.5%) | 2 (7.7%) | 1.00a |
| SRS/SRT | 1 (14.3%) | 0 (0.0%) | 1 (3.8%) | 0.26b |
| Initial SRS/SRT dose (Gy) | 0.71a | |||
| Mean (SD) | 20.6 (1.5) | 20.4 (2.7) | 20.4 (2.4) | |
| Median | 20.0 | 20.0 | 20.0 | |
| Range | (18.0-22.0) | (14.0-27.0) | (14.0-27.0) | |
| Fractions | 1.00b | |||
| SRS (1) | 7 (100.0%) | 18 (94.7%) | 25 (96.2%) | |
| SRT (3) | 0 (0.0%) | 1 (5.3%) | 1 (3.8%) | |
| Met target dosimetric goal | (N = 6) | (N = 10) | (N = 16) | |
| V12 (<10 ccs) or V14(<7 ccs) | 5 | 9 | 14 | 0.70c |
| Total number of courses administered | 0.85a | |||
| Mean (SD) | 2.1 (1.8) | 1.7 (0.7) | 1.8 (1.1) | |
| Median | 2.0 | 2.0 | 2.0 | |
| Range | (1.0-6.0) | (1.0-3.0) | (1.0-6.0) | |
| Systemic therapy prior to radiation | 6 (86%) | 18 (95%) | 24 (92%) | 0.47a |
| Cytotoxic chemotherapy | 3 (43%) | 8 (42%) | 11 (42%) | 1.00a |
| Immunotherapy | 5 (71%) | 17 (90%) | 22 (85%) | 0.29a |
| Targeted therapy | 3 (43%) | 8 (42%) | 11 (42%) | 1.00a |
| Cumulative cytotoxic chemo exposure prior to first radiation (days) | (N = 1) | (N = 3) | (N = 4) | 0.18d |
| Mean (SD) | 122.0 | 98.3 (29.1) | 104.3 (26.6) | |
| Median (IQR) | 122 (122, 122) | 111 (65, 119) | 115 (88, 121) | |
| Range | 122.0, 122.0 | 65.0, 119.0 | 65.0, 122.0 | |
| Cumulative cytotoxic chemo exposure prior to RN (days) | (N = 2) | |||
| Mean (SD) | 148.5 (37.5) | |||
| Median (IQR) | 149 (122, 175) | |||
| Range | 122.0, 175.0 | |||
| Cumulative immunotherapy exposure prior to first radiation (days) | (N = 4) | (N = 13) | (N = 17) | 0.73d |
| Mean (SD) | 130.0 (74.9) | 164.8 (124.8) | 156.6 (113.9) | |
| Median (IQR) | 128 (66, 195) | 120 (63, 245) | 120 (63, 203) | |
| Range | 61.0, 203.0 | 42.0, 378.0 | 42.0, 378.0 | |
| Cumulative immunotherapy exposure prior to RN (days) | (N = 5) | |||
| Mean (SD) | 317.0 (275.3) | |||
| Median (IQR) | 203 (196, 351) | |||
| Range | 61.0, 774.0 | |||
| Cumulative targeted therapy exposure prior to first radiation (days) | (N = 1) | (N = 3) | (N = 4) | 0.18d |
| Mean (SD) | 232.0 | 59.0 (16.1) | 102.3 (87.5) | |
| Median (IQR) | 232 (232, 232) | 61 (42, 74) | 68 (52, 153) | |
| Range | 232.0, 232.0 | 42.0, 74.0 | 42.0, 232.0 | |
| Cumulative targeted therapy exposure prior to RN (days) | (N = 3) | |||
| Mean (SD) | 210.0 (30.6) | |||
| Median (IQR) | 223 (175, 232) | |||
| Range | 175.0, 232.0 | |||
| Number prior systemic treatments | 1.00b | |||
| Mean (SD) | 3.3 (1.6) | 3.9 (2.6) | 3.8 (2.3) | |
| Median | 3.0 | 3.0 | 3.0 | |
| Range | (1.0-6.0) | (1.0-10.0) | (1.0-10.0) | |
| Surgery prior to radiation | 4 (57%) | 5 (26%) | 9 (35%) | 0.19a |
| Surgery to radiation (days) | 0.54b | |||
| Mean (SD) | 44.3 (19.8) | 33.4 (25.5) | 38.2 (22.5) | |
| Median | 49.0 | 23.0 | 30.0 | |
| Range | (18.0-61.0) | (15.0-78.0) | (15.0-78.0) |
Abbreviations: IQR, interquartile range; RN, radiation necrosis; SD, standard deviation; SRS, stereotactic radiosurgery; SRT, Stereotactic Radiotherapy; WBRT, whole brain radiation therapy.
Fisher exact.
Wilcoxon.
Chi-square.
Kruskal-Wallis.
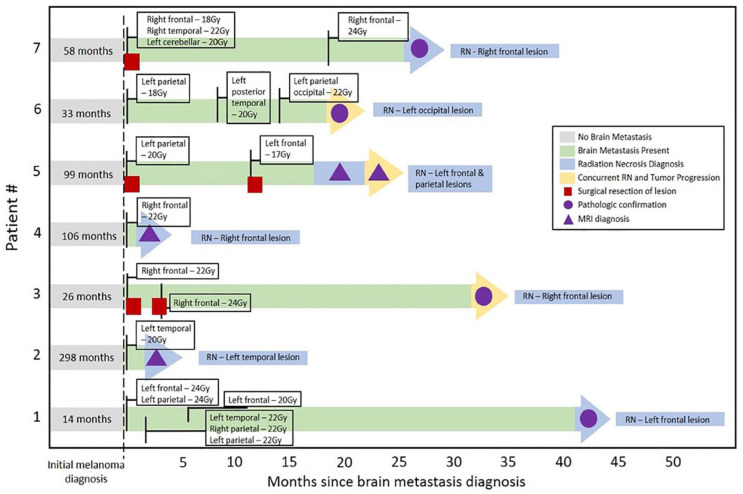
Some patients had radiation therapy for ICM prior to their first course of SRS at our institution, either WBRT at our institution (N = 2) or SRS at an outside institution (N = 1). Most patients received initial SRS in a single fraction (N = 25), with the remainder undergoing Stereotactic radiotherapy in 3 fractions (N = 1). Median dose was 20 Gy (range = 14-27 Gy) and did not differ significantly between patients with and without RN. Many patients underwent multiple courses of SRS (median 2 courses; range 1-6 courses), and/or WBRT (N = 5), and number of treatments was not associated with development of RN (Table 3). There were 16 patients with data evaluable for dosimetric analysis of which 14 met target dosimetric goals (V12 < 10 ccs, V14 < 7 ccs). There was no significant association between patients who met the target dosimetric goal and RN outcome. Individual patient treatment and time to development of RN are detailed in Figure 1.
Figure 1.
Patients with radiation necrosis diagnosis.
Individual patient timelines depict radiation treatment and time to subsequent diagnosis of RN.
RN indicates radiation necrosis.
RN management
In patients who were diagnosed with RN, the median period between the first SRS treatment at our institution and development of RN was 658 (range 48-1152) days.
Table 4 details treatment and outcomes for the 10 instances of RN documented in 7 different patients. Clinical management for these patients included systemic steroids (N = 7), surgery (N = 5), laser ablation (N = 1), and/or no treatment (N = 1). No patient received bevacizumab. Concurrent TP was identified in 4 cases, prompting additional systemic therapy in 2 instances. Improvement in RN was documented in some patients, clinically (N = 2) and/or on brain MRI (N = 4).
Table 4.
Radiation necrosis management and outcomes.
| Patient number | TP | Steroids | Surgery | Laser ablation | Bevacizumab | Systemic therapy | RN treatment outcome |
|---|---|---|---|---|---|---|---|
| 1 | N | Y | Y | N | N | N | Improvement—lesion and clinical |
| 2 | N | N | N | N | N | N | Not applicable |
| 2 | N | Y | N | N | N | N | Progression |
| 3 | N | Y | N | N | N | N | Stable |
| 3 | Y | Y | Y | N | N | N | Progression |
| 3 | Y | Y | Y | N | N | Y | Progression |
| 4 | N | Y | N | N | N | N | Improvement—lesion and clinical |
| 5 | Y | Y | N | Y | N | N | Improvement—lesion only |
| 6 | Y | N | Y | N | N | Y | Progression |
| 7 | N | N | Y | N | N | N | Improvement—lesion only |
Abbreviations: N, no; RN, radiation necrosis; TP, concurrent tumor progression; Y, yes.
Descriptions under radiation necrosis treatment outcome refer to stable, progressive, or improved radiation necrosis.
Outcomes
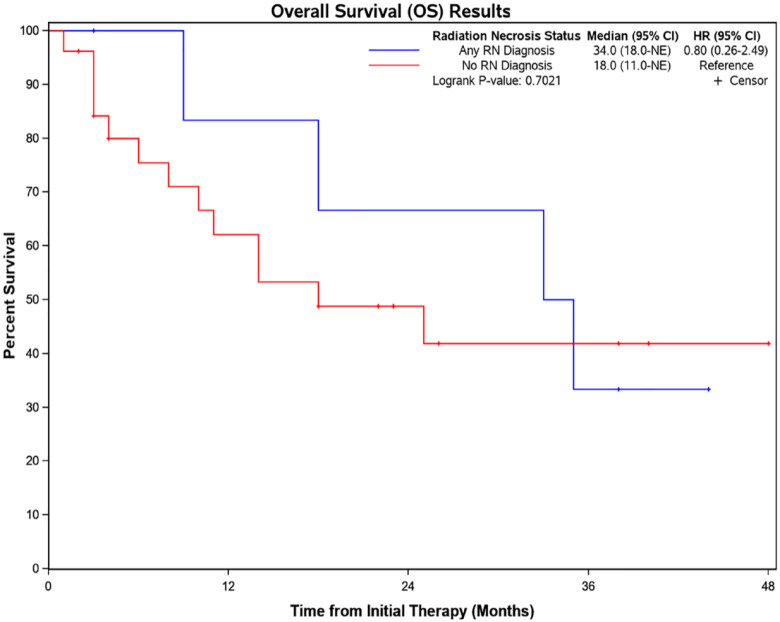
At the time of record review 9 of 26 patients included were alive (36%). There was no significant difference in overall survival between those with and without RN (Figure 2). Mean follow-up time from first SRS treatment at our institution was 27 months (median 16 months; range = 1-82 months). Follow-up was longer for patients with a diagnosis of RN compared with those without RN (mean 44 vs 21 months), although this difference was not statistically significant when Kaplan-Meier analysis was undertaken with RN as a time-dependent covariate. Factors cited in eventual death were systemic disease (50%; N = 13), central nervous system (CNS) disease (31%; N = 8), and treatment complications (12%; N = 3).
Figure 2.
Overall survival.
Kaplan-Meier estimate of overall survival in patients with and without radiation necrosis.
CI indicates confidence interval; HR, hazard ratio; RN, radiation necrosis.
Discussion
With tumor biology established as a driver of ICM behavior and response to treatment, clinical decisions to address ICM may increasingly be influenced by a patient’s primary malignancy; melanoma, due to its clinical aggressiveness, is a suitable cancer type for studying this. In this retrospective analysis, we reviewed 26 consecutive patients with ICM from melanoma to understand the risk and experience of RN following treatment with SRS. In most cases and similar to other studies of RN following SRS, our data set contained limited samples with pathologic confirmation of RN.13,16,19 Thus, treatment decisions were based on best clinical judgment of TP versus RN. The lack of uniform diagnostic criteria for RN remains a persistent challenge in the study and clinical management of radiation complications.
In our study, 27% of patients received a diagnosis of RN after SRS, consistent with the higher end of the range reported in the literature.12,14,17,19 This relatively large percentage may represent differences in our institutional practice for the diagnosis of RN as well as longer follow-up of lesions that subsequently developed RN. In practice, the determination of RN was often made after retrospective recognition that TP was unlikely, given the patient’s overall disease state, time course, and symptoms; the actual rates may also be clinically underestimated as a result.
The interval between SRS and RN diagnosis in this study ranged from 1.6 to 38 months. This delay between treatment and development of toxicity is consistent with other studies.15,31 In this study, the follow-up time for patients with a diagnosis of RN was more than double on average compared with those without diagnosed RN, which presumably reflects the likelihood that patients who live longer have a greater chance of developing RN at some point. These data support the practice that patients with metastatic melanoma who have been treated with SRS for ICM should be followed for years after radiation, and we should maintain low thresholds of clinical suspicion and further evaluation for RN if new symptoms develop.
The median overall survival in patients with RN diagnosis was 35 months, compared with 18 months in patients without a diagnosis of RN. This reflects that patients with melanoma live longer both preceding and following the diagnosis of ICM compared with older studies, which likely is a direct result of advances in systemic therapy in combination with improved radiation techniques providing more effective overall disease control.32 Importantly, as patients live longer, the cumulative risk of RN may continue to increase.
We did not identify any statistically significant risk factors for RN, however, did observe a trend that is in keeping with other studies whereby large ICM size and prior surgical resection are associated risk factors for the subsequent development of RN.15,25-27 Interestingly, in patients who were treated with surgical resection, the interval prior to starting adjuvant SRS was higher in the group who ultimately developed RN. This suggests timing of adjuvant SRS is a potential risk factor that merits further study on a larger scale for confirmation. Given the low number of patients with a diagnosis of RN, we were not able to draw any conclusions on fractional dose of radiation received per lesion as a risk factor; however, radiation doses prescribed are inversely related to lesion size which was identified as a potential risk factor in this study, and thus may indirectly associate a higher risk of RN with fractionated radiation dosing required for larger lesions. As other studies have shown the risk of radionecrosis to be dose-dependent, some therapeutic strategies using de-escalated doses and subsequent surgery (neoadjuvant SRS) or staged dosing over some weeks (staged SRS) might be safer options than definitive SRS in certain conditions.33,34 In addition, since the radionecrosis risk depends on the proportion of the healthy brain tissue exposed to high radiation doses, some SRS techniques, which are characterized by greater dose conformity than that in our study, might determine better outcomes than ours.35-37 However, this assumption should be addressed in specific trials.
Potentially related to a small cohort, we did not elucidate any trends in systemic therapies preceding RN diagnosis. Other reports have indicated that preceding capecitabine treatment was associated with increased risk of RN while use of checkpoint inhibitors, BRAF inhibitors, or other novel targeted therapies prior to SRS did not increase the risk of developing RN in most studies.14,26,38-41 One report however did link BRAF inhibition to an increased risk of RN following SRS.42 It remains unclear whether certain chemotherapy regimens are more likely than others to be related to the risk of RN.
Our study did not demonstrate an association between tumor somatic alteration status and risk of RN. In comparison, previous studies in melanoma patients support a mixed conclusion regarding the association of BRAF mutation and the risk of RN. For example, the presence of BRAF mutation was independently protective against RN in a cohort of 195 melanoma patients evaluated as part of a larger study of brain metastases and RN.14 Separately, a more recent study of 203 patients with melanoma treated with concurrent or nonconcurrent SRS and checkpoint inhibition found the presence of BRAF mutation to be associated with an increased RN risk, including symptomatic RN.43 These findings highlight the need for further study into how the mutational landscape across tumors confers susceptibility to SRS outcomes. Moreover, while BRAF and NRAS mutations are somatic events in melanoma, it is possible that germline genetics may be more relevant to the development of RN. Our cohort does not include data to analyze this question; however, inclusion of germline data will be a future subject of study, particularly for those genes thought to be related to radiation sensitivity, including DNA repair pathway mediators involved in homologous recombination and nonhomologous end joining.44
We previously published a study on RN following SRS in patients with ICM from lung cancer.15 In that cohort of patients, treatment was undertaken for symptomatic RN in 80% of cases and resulted in clinical improvement 75% of the time. In the current cohort of patients with ICM from melanoma, RN was treated in 90% of cases, however clinical improvement was only achieved 20% of the time (N = 2). In one patient this was achieved with steroid therapy alone, while the other patient required both steroids and surgical resection. A possible explanation to the lack of clinical benefit following treatment of RN in our cohort is that patients with radiographic progression following treatment of RN may have represented a potential mixed concurrent TP with RN. Regardless, our results show that RN from melanoma is difficult to manage successfully and thus strategies to mitigate the development of RN following treatment of ICM with SRS is warranted.
None of the patients in our cohort were treated with bevacizumab for management of RN, which has been shown in a small randomized controlled trial to be associated with radiological treatment response.45 This may be due to treating physicians’ desire to avoid using bevacizumab, which carries a substantial risk of hemorrhage, in cases of ICM from melanoma, which are more likely to bleed compared with ICM from other primary tumors.46,47
This study is limited by its retrospective design, small sample size, homogenous population, and, in some patients, short follow-up period. Larger, more inclusive prospective studies are needed to understand the risk of RN following SRS for ICM from melanoma, and to inform associated clinical decision-making.
Conclusion
There is a substantial rate of RN in patients with metastatic melanoma who undergo SRS for treatment of ICM. It remains difficult to predict who will develop RN and indeed this study did not reveal any statistically significant risk factors. However, there were trends toward larger lesions, prior surgical resection, and a longer period between surgery and adjuvant SRS in patients with intracranial melanoma who developed RN. Additional work is warranted to discern whether certain chemotherapy regimens convey increased risk of subsequent RN development and whether germline genetics known to influence radiosensitivity play a role in the progression of RN. Once RN has been diagnosed and deemed clinically significant in patients with melanoma, it is quite difficult to treat successfully. Prospective study of RN risk and treatment is needed in patients with ICM from melanoma to improve clinical outcomes.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Steven Schild, MD has served as an author and editor of UpToDate, Inc. Alan Bryce, MD discloses participation on a Data Safety Monitoring Board or Advisory Board at Clovis Oncology as well as payment/honoraria by Merck & Co., Inc., Astellas Pharma Inc., and Pfizer Inc. for lectures, presentations, speakers’ bureaus, manuscript writing or educational events. Terence Sio, MD, MS provides strategic and scientific recommendations as a member of the Advisory Board and speaker for Novocure, Inc., which is not in any way associated with the content or disease site as presented in this manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: Study design: AS, and ABP.
Study implementation: AS, SES, JBA, RSZ, NPP, AHB, SAV, TTS, and ABP.
Data collection: HMT, SPFE, VSE, AS, TTS, and ABP.
Data analysis and interpretation: HMT, SPFE, RJB, TTS, and ABP.
Manuscript drafting: HMT and SPFE.
Manuscript revisions and approval: HMT, SPFE, VSE, AS, RJB, SES, JBA, RSZ, NPP, AHB, SAV, TTS, and ABP.
Ethics Approval and Consent to Participate: This study was approved as minimal risk under Mayo Clinic Institutional Review Board ID# 18-003868, without further requirement for ethics committee review. Date of initial study approval: 5/7/2018.
ORCID iDs: Shannon P Fortin Ensign  https://orcid.org/0000-0002-7433-528X
https://orcid.org/0000-0002-7433-528X
Alan H Bryce  https://orcid.org/0000-0002-0206-3895
https://orcid.org/0000-0002-0206-3895
References
- 1. Sawaya R, Bindal RK, Lang FF, et al. Metastatic brain tumors. In: Kaye EL, ed. Brain Tumors: An Encyclopedic Approach. 2nd ed. Churchill Livingstone; 2001:999-1026. [Google Scholar]
- 2. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed(1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865-2872. doi: 10.1200/JCO.2004.12.149 [DOI] [PubMed] [Google Scholar]
- 3. Fidler IJ, Schackert G, Zhang RD, Radinsky R, Fujimaki T. The biology of melanoma brain metastasis. Cancer Metastasis Rev. 1999;18:387-400. doi: 10.1023/a:1006329410433 [DOI] [PubMed] [Google Scholar]
- 4. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807-810. doi: 10.1016/0002-9610(78)90171-x [DOI] [PubMed] [Google Scholar]
- 5. Budman DR, Camacho E, Wittes RE. The current causes of death in patients with malignant melanoma. Eur J Cancer (1965). 1978;14:327-330. doi: 10.1016/0014-2964(78)90201-3 [DOI] [PubMed] [Google Scholar]
- 6. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117:1687-1696. doi: 10.1002/cncr.25634 [DOI] [PubMed] [Google Scholar]
- 7. Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control. 2009;16:248-255. doi: 10.1177/107327480901600307 [DOI] [PubMed] [Google Scholar]
- 8. Franchino F, Rudà R, Soffietti R. Mechanisms and therapy for cancer metastasis to the brain. Front Oncol. 2018;8:161. doi: 10.3389/fonc.2018.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meier S, Baumert BG, Maier T, et al. Survival and prognostic factors in patients with brain metastases from malignant melanoma. Onkologie. 2004;27:145-149. doi: 10.1159/000076903 [DOI] [PubMed] [Google Scholar]
- 10. Ferini G, Viola A, Valenti V, et al. Whole brain irradiation or stereotactic radiosurgery for five or more brain metastases (WHOBI-STER): a prospective comparative study of neurocognitive outcomes, level of autonomy in daily activities and quality of life. Clin Transl Radiat Oncol. 2022;32:52-58. doi: 10.1016/j.ctro.2021.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bagshaw HP, Ly D, Suneja G, Jensen RL, Shrieve DC. Local control of melanoma brain metastases treated with stereotactic radiosurgery. J Radiosurg SBRT. 2016;4:181-190. [PMC free article] [PubMed] [Google Scholar]
- 12. Williams BJ, Suki D, Fox BD, et al. Stereotactic radiosurgery for metastatic brain tumors: a comprehensive review of complications. J Neurosurg. 2009;111:439-448. doi: 10.3171/2008.11.JNS08984 [DOI] [PubMed] [Google Scholar]
- 13. Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87:449-457. doi: 10.1016/j.ijrobp.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 14. Miller JA, Bennett EE, Xiao R, et al. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys. 2016;96:1060-1069. doi: 10.1016/j.ijrobp.2016.08.039 [DOI] [PubMed] [Google Scholar]
- 15. Sharma A, Mountjoy LJ, Butterfield RJ, et al. Expanding the spectrum of radiation necrosis after stereotactic radiosurgery (SRS) for intracranial metastases from lung cancer: a retrospective review. Am J Clin Oncol. 2020;43:128-132. doi: 10.1097/COC.0000000000000642 [DOI] [PubMed] [Google Scholar]
- 16. Schuttrumpf LH, Niyazi M, Nachbichler SB, et al. Prognostic factors for survival and radiation necrosis after stereotactic radiosurgery alone or in combination with whole brain radiation therapy for 1-3 cerebral metastases. Radiat Oncol. 2014;9:105. doi: 10.1186/1748-717X-9-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94:899-904. doi: 10.3171/jns.2001.94.6.0899 [DOI] [PubMed] [Google Scholar]
- 19. Sharma M, Jia X, Ahluwalia M, et al. First follow-up radiographic response is one of the predictors of local tumor progression and radiation necrosis after stereotactic radiosurgery for brain metastases. Cancer Med. 2017;6:2076-2086. doi: 10.1002/cam4.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz RB, Holman BL, Polak JF, et al. Dual-isotope single-photon emission computerized tomography scanning in patients with glioblastoma multiforme: association with patient survival and histopathological characteristics of tumor after high-dose radiotherapy. J Neurosurg. 1998;89:60-68. doi: 10.3171/jns.1998.89.1.0060 [DOI] [PubMed] [Google Scholar]
- 21. Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol. 2010;99:81-88. doi: 10.1007/s11060-009-0106-z [DOI] [PubMed] [Google Scholar]
- 22. Sugahara T, Korogi Y, Tomiguchi S, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000;21:901-909. [PMC free article] [PubMed] [Google Scholar]
- 23. Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96:191-197. doi: 10.1002/ijc.1016 [DOI] [PubMed] [Google Scholar]
- 24. Omay SB, Atsina KK, Baehring JM. Chapter 53—nonneoplastic mass lesions of the central nervous system. In: Newton HB, ed. Handbook of Neuro-Oncology Neuroimaging. 2nd ed. Elsevier; 2016:653-665. [Google Scholar]
- 25. Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996-1001. doi: 10.1016/j.ijrobp.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 26. Sneed PK, Mendez J, Vemer-van den Hoek JG, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123:373-386. doi: 10.3171/2014.10.JNS141610 [DOI] [PubMed] [Google Scholar]
- 27. Mohammadi AM, Schroeder JL, Angelov L, et al. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg. 2017;126:735-743. doi: 10.3171/2016.3.JNS153014 [DOI] [PubMed] [Google Scholar]
- 28. Inoue HK, Sato H, Seto K, et al. Five-fraction CyberKnife radiotherapy for large brain metastases in critical areas: impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res. 2014;55:334-342. doi: 10.1093/jrr/rrt127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korytko T, Radivoyevitch T, Colussi V, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64:419-424. doi: 10.1016/j.ijrobp.2005.07.980 [DOI] [PubMed] [Google Scholar]
- 30. Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med. 2009;37:2939-2945. doi: 10.1097/CCM.0b013e3181b7fbbb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291-298. doi: 10.1016/s0360-3016(99)00507-6 [DOI] [PubMed] [Google Scholar]
- 32. Schild SE, Behl D, Markovic SN, et al. Brain metastases from melanoma: is there a role for concurrent temozolomide in addition to whole brain radiation therapy? Am J Clin Oncol. 2010;33:633-636. doi: 10.1097/COC.0b013e3181c4c54b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ginalis EE, Cui T, Weiner J, Nie K, Danish S. Two-staged stereotactic radiosurgery for the treatment of large brain metastases: single institution experience and review of literature. J Radiosurg SBRT. 2020;7:105-114 [PMC free article] [PubMed] [Google Scholar]
- 34. Palmisciano P, Ferini G, Khan R, et al. Neoadjuvant stereotactic radiotherapy for brain metastases: systematic review and meta-analysis of the literature and ongoing clinical trials. Cancers (Basel). 2022;14:4328. doi: 10.3390/cancers14174328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao H, Xiao Z, Zhang Y, et al. Dosimetric comparisons of different hypofractionated stereotactic radiotherapy techniques in treating intracranial tumors > 3 cm in longest diameter. J Neurosurg. 2019;132:1024-1032. doi: 10.3171/2018.12.JNS181578 [DOI] [PubMed] [Google Scholar]
- 36. Inserra F, Barone F, Palmisciano P, et al. Hypofractionated gamma knife radiosurgery: institutional experience on benign and malignant intracranial tumors. Anticancer Res. 2022;42:1851-1858. doi: 10.21873/anticanres.15661 [DOI] [PubMed] [Google Scholar]
- 37. Potrebko PS, Keller A, All S, et al. GammaKnife versus VMAT radiosurgery plan quality for many brain metastases. J Appl Clin Med Phys. 2018;19:159-165. doi: 10.1002/acm2.12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gagliano A, Prestifilippo A, Cantale O, et al. Role of the combination of cyclin-dependent kinase inhibitors (CDKI) and radiotherapy (RT) in the treatment of metastatic breast cancer (MBC): advantages and risks in clinical practice. Front Oncol. 2021;11:643155. doi: 10.3389/fonc.2021.643155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kotecha R, Miller JA, Venur VA, et al. Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurg. 2018;129:50-59. doi: 10.3171/2017.1.JNS162797 [DOI] [PubMed] [Google Scholar]
- 40. Gaudy-Marqueste C, Carron R, Delsanti C, et al. On demand gamma-knife strategy can be safely combined with BRAF inhibitors for the treatment of melanoma brain metastases. Ann Oncol. 2014;25:2086-2091. doi: 10.1093/annonc/mdu266 [DOI] [PubMed] [Google Scholar]
- 41. Xu Z, Lee CC, Ramesh A, et al. BRAF V600E mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases. J Neurosurg. 2017;126:726-734. doi: 10.3171/2016.2.JNS1633 [DOI] [PubMed] [Google Scholar]
- 42. Patel KR, Chowdhary M, Switchenko JM, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res. 2016;26:387-394. doi: 10.1097/CMR.0000000000000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lehrer EJ, Gurewitz J, Bernstein K, et al. Concurrent administration of immune checkpoint inhibitors and stereotactic radiosurgery is well-tolerated in patients with melanoma brain metastases: an international multicenter study of 203 patients. Neurosurgery. 2022;91:872-882. doi: 10.1227/neu.0000000000002127 [DOI] [PubMed] [Google Scholar]
- 44. Chistiakov DA, Voronova NV, Chistiakov PA. Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients. Acta Oncol. 2008;47:809-824. doi: 10.1080/02841860801885969 [DOI] [PubMed] [Google Scholar]
- 45. Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15:1257-1263. doi: 10.1093/neuonc/not085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (avastin(R)) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017 [DOI] [PubMed] [Google Scholar]
- 47. Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep. 2012;14:373-381. doi: 10.1007/s11883-012-0250-3 [DOI] [PubMed] [Google Scholar]