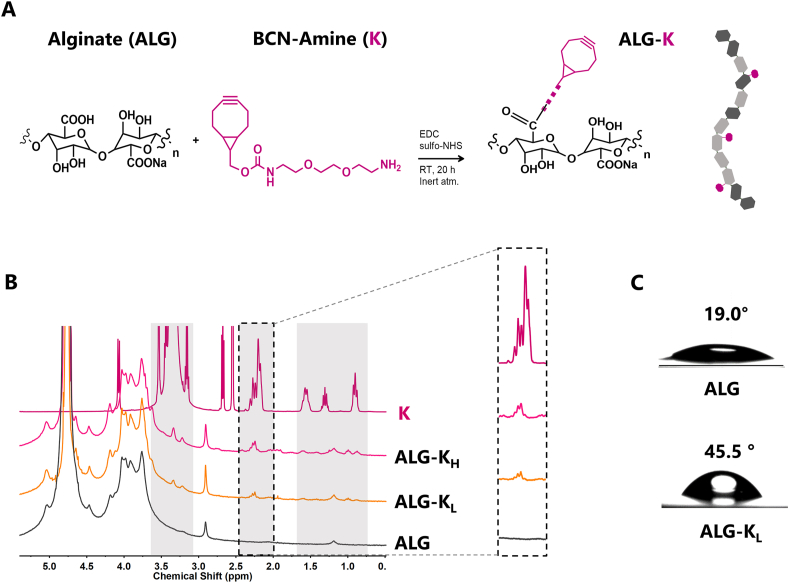
Fig. 1.
Synthesis of amphiphilic cyclooctyne-modified alginate derivatives is efficient and reproducible. A) Schematic representation of alginate-cyclooctyne derivative (ALG-K) production by BCN-amine (K) incorporation onto ALG carboxyl groups via carbodiimide chemistry. B) 1H NMR of ALG-K derivatives with low (ALG-KL; MD = 2.7 ± 0.4%) and high (ALG-KH; MD = 5.1 ± 0.8%) modification degrees (MD, average values from n ≥ 4 independent modification batches). Gray regions show characteristic K peaks, and the inset shows the one used for MDs calculation. C) Optical contact angles of ALG and ALG-KL films.