Abstract
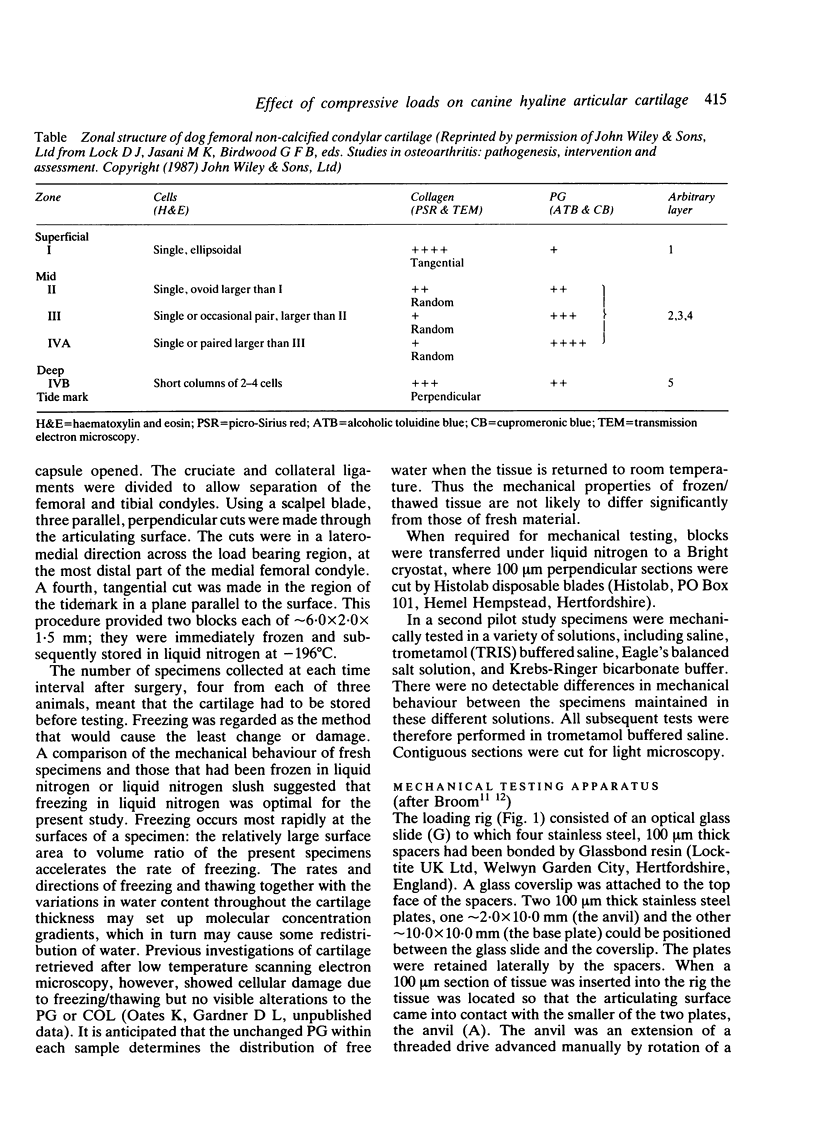
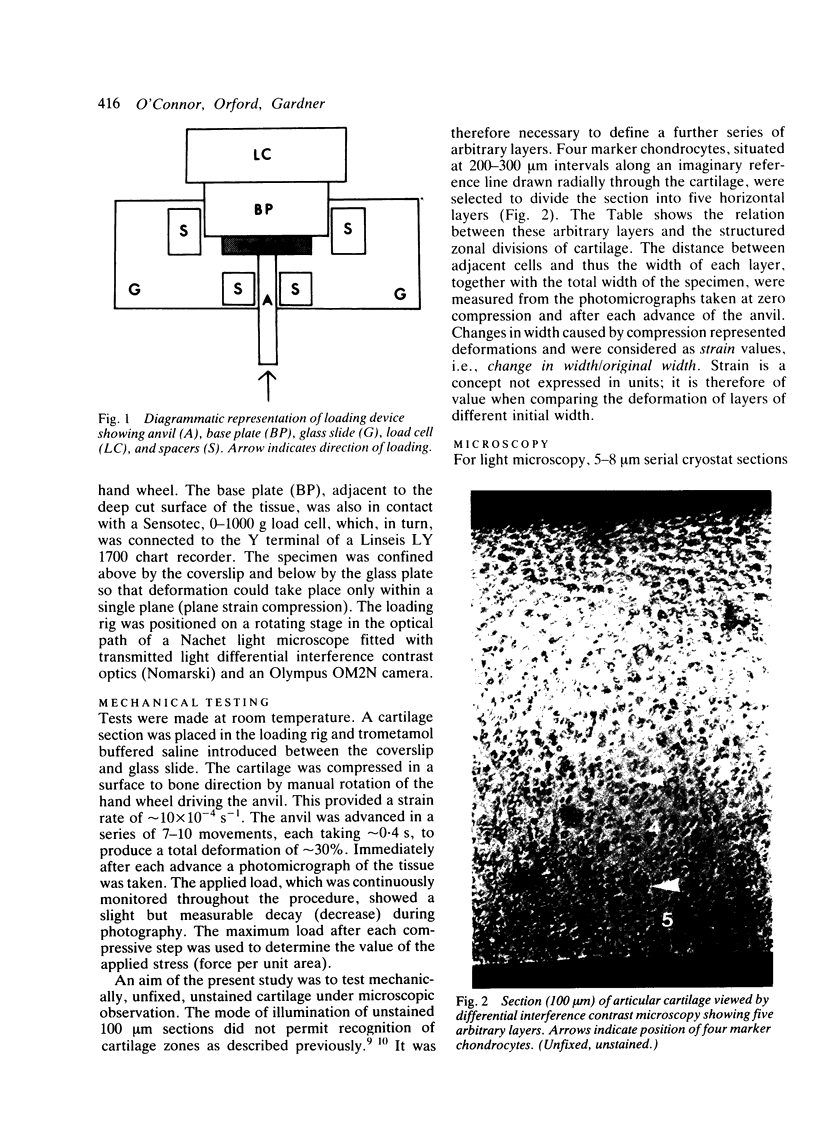
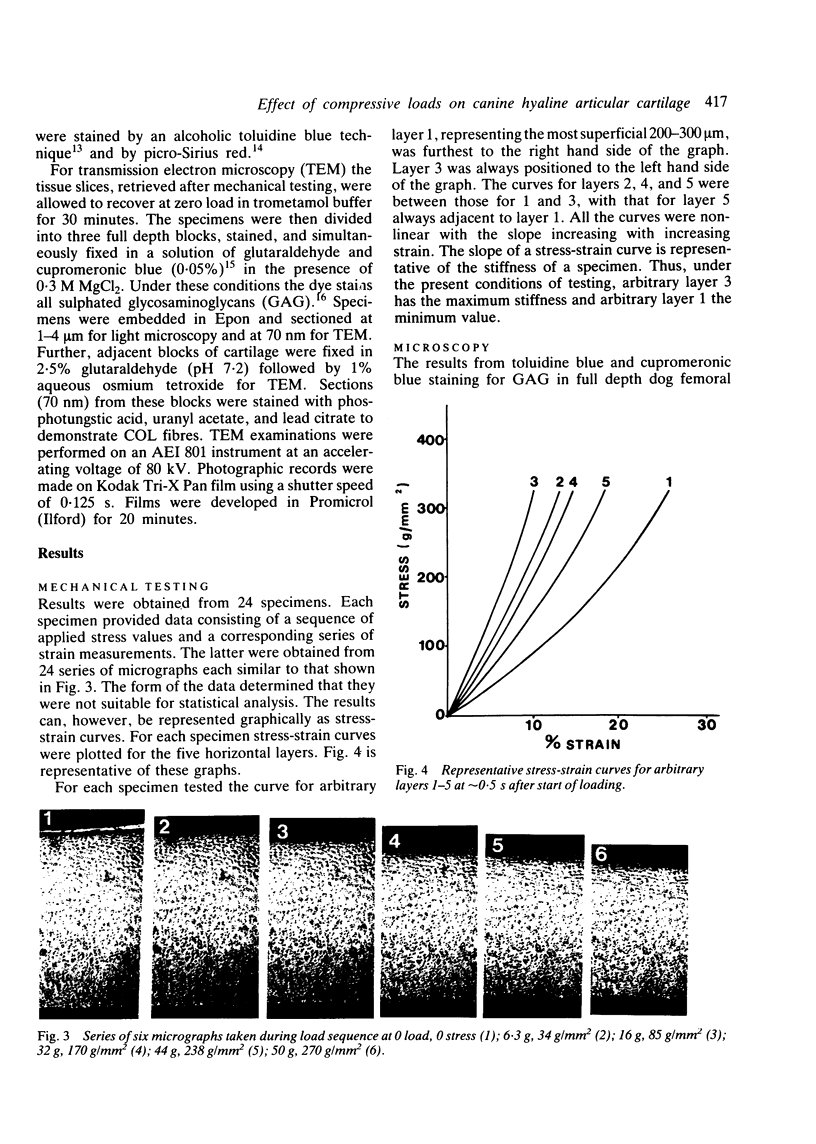
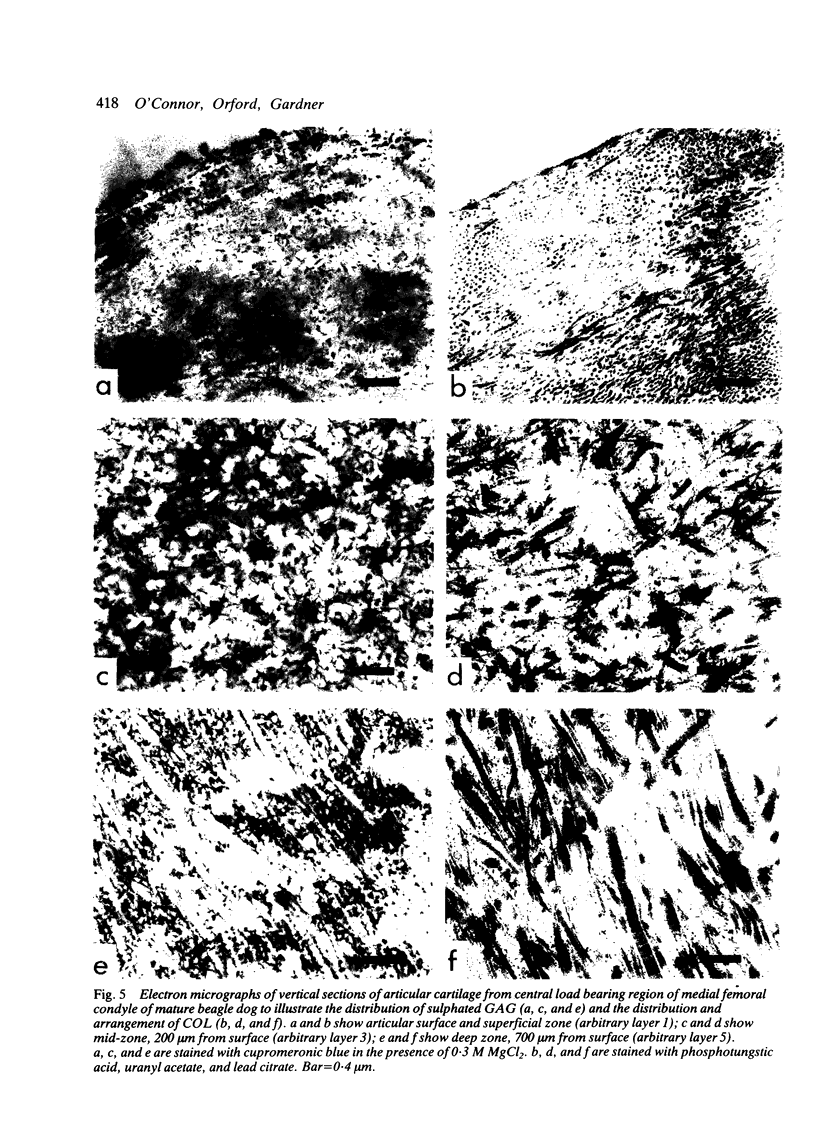
Thin (100 micron) perpendicular slices of canine femoral condylar cartilage were placed horizontally on the stage of a Nachet microscope and viewed by transmitted light in the differential interference contrast mode. Each slice was held on the microscope stage by a loading rig and tested mechanically in compression. Measured loads to a maximum of approximately 2-3 MN/m2 were applied to the end of the slice corresponding to the articular surface. Photographs were taken of the cartilage before and during loading, and the distance by which selected chondrocytes were displaced was used as an index of mechanical strain, i.e., of change in length/original length. Maximum strains were observed in the superficial cartilage zone. Minimum strains were recorded in the mid-zone, at a depth corresponding to approximately 75% of the total cartilage thickness. The relative concentrations of cartilage collagen (COL) and proteoglycan (PG) were assessed by the light and electron microscopic histochemical study of cartilage sections taken from contiguous blocks. Superficial cartilage, which deformed most, had high concentrations of oriented COL fibres, low concentrations of PG. Mid-zone cartilage, which deformed least, had lower concentrations of randomly arrayed COL fibres but relatively high concentrations of PG.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. G., Bahrani A. S., Gardner D. L. In vitro measurement of articular cartilage deformations in the intact human hip joint under load. J Bone Joint Surg Am. 1979 Jul;61(5):744–755. [PubMed] [Google Scholar]
- Broom N. D., Marra D. L. Ultrastructural evidence for fibril-to-fibril associations in articular cartilage and their functional implication. J Anat. 1986 Jun;146:185–200. [PMC free article] [PubMed] [Google Scholar]
- Broom N. D., Myers D. B. A study of the structural response of wet hyaline cartilage to various loading situations. Connect Tissue Res. 1980;7(4):227–237. doi: 10.3109/03008208009152358. [DOI] [PubMed] [Google Scholar]
- Day W. H., Swanson S. A., Freeman M. A. Contact pressures in the loaded human cadaver hip. J Bone Joint Surg Br. 1975 Aug;57(3):302–313. [PubMed] [Google Scholar]
- O'Connor P., Oates K., Gardner D. L., Middleton J. F., Orford C. R., Brereton J. D. Low temperature and conventional scanning electron microscopic observations of dog femoral condylar cartilage surface after anterior cruciate ligament division. Ann Rheum Dis. 1985 May;44(5):321–327. doi: 10.1136/ard.44.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford C. R., Gardner D. L., O'Connor P., Bates G., Swallow J. J., Brito-Babapulle L. A. Ultrastructural alterations in glycosaminoglycans of dog femoral condylar cartilage after surgical division of an anterior cruciate ligament: a study with cupromeronic blue in a critical electrolyte concentration technique. J Anat. 1986 Oct;148:233–244. [PMC free article] [PubMed] [Google Scholar]
- Poole A. R. The relationship between toluidine blue staining and hexuronic acid content of cartilage matrix. Histochem J. 1970 Sep;2(5):425–430. doi: 10.1007/BF01004723. [DOI] [PubMed] [Google Scholar]





