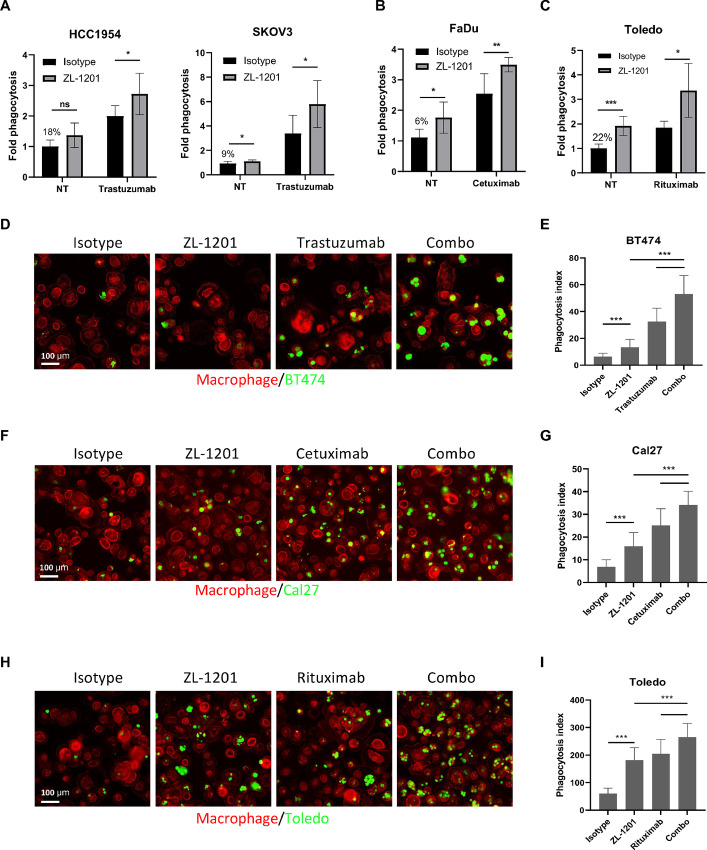
FIGURE 2.
ZL-1201 synergizes with mAbs for potent tumor cell elimination. A,In vitro phagocytosis of HER2-amp solid tumor cells including HCC1954 and SKOV3 treated with isotype control (10 μg/mL), ZL-1201 (10 μg/mL), trastuzumab (0.1 μg/mL), or their combination. B,In vitro phagocytosis of EGFR-driven FaDu HNSCC cells treated with isotype control (10 μg/mL), ZL-1201 (10 μg/mL), cetuximab (0.1 μg/mL), or their combination. C,In vitro phagocytosis of CD20-expressing Toledo lymphoma cells treated with isotype control (10 μg/mL), ZL-1201 (10 μg/mL), rituximab (0.1 μg/mL), or their combination as indicated. Fold phagocytosis in A and B was calculated relative to isotype in nontreated (NT) conditions, the raw phagocytosis percentages are indicated in each figure. Data were shown as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. In vitro phagocytosis was performed in in vitro coculture system using macrophage (red), BT474 (D), Cal27 (F), and Toledo (H) (green) cancer cells and photographed by confocal imaging. E, G, and I, Phagocytosis index was calculated from D, F, and H. Data were shown as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.