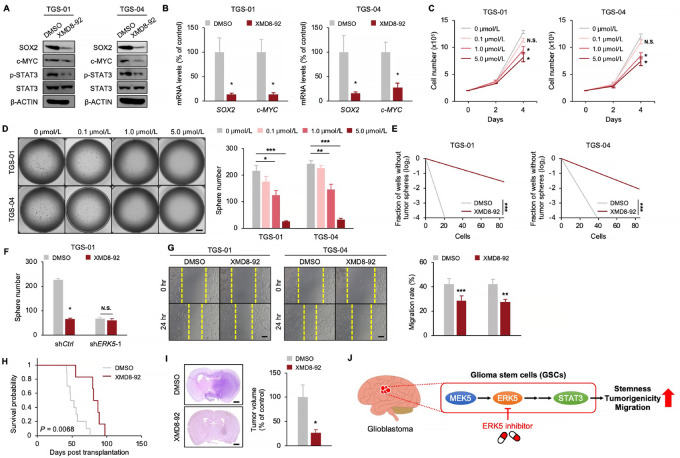
FIGURE 6.
The pharmacologic inhibition of ERK5 suppresses GSC phenotypes. TGS-01 and TGS-04 cells were treated with XMD8-92, followed by determination of protein levels of SOX2, c-MYC, p-STAT3, and STAT3 (β-ACTIN served as the loading control; A), mRNA levels of SOX2 and c-MYC (n = 4, mean ± SE; *, P < 0.05; B), cell viability (n = 4, mean ± SE; *, P < 0.05; C), tumorsphere number (n = 10, mean ± SE; *, P < 0.05; **, P < 0.01; ***, P < 0.001; D), stem cell frequency by in vitro limiting dilution assay (estimated frequencies of clonogenic cells in GSC tumorspheres were calculated by ELDA; ***, P < 0.001; E). F, TGS-01 cells were infected with shERK5 in combination with XMD8-92 treatment at 5.0 μmol/L followed by determination of tumorsphere number (n = 5, mean ± SE; *, P < 0.05). Migration ability in TGS-01 cells infected with shERK5 (n = 4, mean ± SE; **, P < 0.01; ***, P < 0.001; G), Kaplan–Meier survival analysis of mice inoculated with TGS-01 cells pre-treated with 5.0 μmol/L KY-065 for 4 days (n = 6–10; H). P value was calculated using a log-rank test. I, Histologic analyses of brains dissected at 40 days after intracranial transplantation. Tissue sections were stained with H&E (n = 5, mean ± SE; *, P < 0.05). J, Schematic model of the findings of this study. MEK5-ERK5-STAT3 axis in GSCs promotes stemness, tumorigenicity, and migration to enhance GBM malignancy. Pharmacologic inhibition of ERK5 by XMD8-92 inhibits this pathway, providing a potential strategy for GSC-directed therapy. Scale bars, 1 mm (D and I) and 100 μm (G).