Abstract
Background
Bacillus Calmette–Guerin (BCG) maintenance therapy is the standard adjuvant treatment of high- and intermediate-risk non-muscle-invasive bladder cancer (NMIBC). However, the problems of shortages and the adverse effects, both local and systemic, that it causes lead to the search for alternatives with devices that improve the penetration of intravesical chemotherapeutics.
Materials and methods
Prospective observational study was conducted from August 2018 to August 2022. Patients diagnosed with intermediate and high-risk NMIBC without CIS who received one of the following three treatments were included: BCG in induction protocol with six weekly instillations and maintenance with three weekly instillations at months 3, 6, and 12. MMC was applied by Physionizer® 30 device with a current of 20 mA for 30 min was used in an induction protocol of 6 weekly instillations followed by 6 monthly instillations as maintenance (EMDA group). MMC was applied by COMBAT BRS System V2.0 device at 43 ± 0.5 ℃ for 60 min was used in an induction protocol of 6 weekly instillations followed by 6 monthly instillations as maintenance (HIVEC group). The primary objective was to compare the 24-month recurrence-free rate between the three groups. The secondary objectives were to evaluate the rate free of progression at 24 months and the degree of toxicity of the treatments.
Results
One hundred and eighty-three patients divided into a HIVEC group with sixty-one patients, EMDA group with fifty-nine patients, and BCG group with sixty-three patients. After a mean follow-up of 25 months (IQR 13–36), the 24-month recurrence-free rate was 82.1% for HIVEC, 80% for EMDA, and 84.6% for BCG (p > 0.05), and a progression-free rate at 24 months of 95.6% for HIVEC, 98.3% for EMDA, and 92.9% for BCG (p > 0.05). No statistically significant differences were found between the three groups with respect to the degree of reported adverse events.
Conclusion
Adjuvant treatment with BCG or MMC applied with COMBAT or EMDA does not present differences in the recurrence-free rate and progression at 24 months in our population of patients with intermediate- and high-risk NMBC without CIS.
Keywords: Bladder cancer, Intravesical administration, Mitomycin C, Bacillus Calmette-Guerin, Hyperthermia, Electromotive force
Introduction
Non-muscle-invasive bladder cancer (NMIBC) represents approximately 75% of bladder cancer diagnoses. Transurethral resection of the bladder (v-TUR) allows the diagnosis and treatment of these tumors. However, NMIBC presents a risk of recurrence and progression to a muscle-invasive form. To predict the risk of recurrence and progression, patients are classified into low-, intermediate-, and high-risk groups (Sylvester et al. 2021; Malmström et al. 2009). BCG maintenance treatment is considered the best therapy in high- and intermediate-risk patients (Oddens et al. 2013; Schmidt et al. 2020; Sharma et al. 2020). However, recent studies suggest that BCG maintenance therapy in all these patients is not a cost-effective alternative (Brausi et al. 2014). In addition, the application of BCG causes both local and systemic adverse effects (Ojea et al. 2007; Tan and Kelly 2018). For all these reasons, alternatives to treatment using devices that improve the penetration of intravesical chemotherapeutics are being sought (Arends et al. 2016).
Advances in the treatment of NMIBC have focused on optimizing the penetration and potentiating the effects of chemotherapy using device-assisted technology.
The three most widely used devices are the radiofrequency-induced thermotherapeutic effect (RITE), which has the largest amount of literature (Tan et al. 2019; Colombo et al. 2003; Guerrero-Ramos et al. 2022), hyperthermic intravesical chemotherapy—HIVEC—using closed recirculation systems (Plata et al. 2021; Sousa et al. 2016; Colombo et al. 2001), and electromotive drug administration (EMDA) (Stasi et al. 2003; Racioppi et al. 2018; Stasi and Riedl 2009). All have been postulated as effective alternatives to BCG treatment. However, the optimal regimen of standard or device-assisted chemotherapy instillations has not been identified, nor has it been determined which patient profile would benefit most from these treatments.
We present a prospective observational study comparing treatment with mitomycin C (MMC) delivered by the EMDA device versus MMC delivered by conduction hyperthermia-HIVEC with the COMBAT device versus BCG in the treatment of patients with intermediate- and high-risk NIVT without carcinoma in situ (CIS).
Materials and methods
Studies design and participants
We conducted a prospective observational study from August 2018 to August 2022. Patients with a histological diagnosis of intermediate- and high-risk urothelial NMIBC without CIS were included. Patients with primary or recurrent bladder cancer were included provided they had not received treatment with BCG or MMC applied with EMDA or HIVEC in the previous 2 years.
Patients with a history of prior CIS or concomitant CIS, a history of stage T2 bladder cancer, non-urothelial carcinomas, or urothelial carcinoma in the upper urinary tract at the time of diagnosis were excluded. Exclusion criteria also applied to the following: allergy to MMC, intolerance, or contraindication to BCG; alterations in the complete blood count or alterations in renal or hepatic function; minor bladder capacity to 150 ml; pregnant/lactating women, and; treatment with chemotherapy or pelvic radiotherapy during the last three months.
The study obtained the approval of the Local Ethics Committee before the experiment was started (IRB number 0992-N-18). It has been conducted in accordance with the principles set forth in the Helsinki Declaration and all patients signed an informed consent accepting the treatment.
Intervention and treatment schedules
Prior to enrollment, urinary cytology, cystoscopy, and resection of all visible bladder tumors were performed. Random biopsies of the bladder mucosa were taken in cases with previous positive cytology, non-papillary tumor, or a history of high-grade tumor.
A second TURBT was performed with an interval of 2–4 weeks when tumor resection was incomplete or there was no muscle in the sample (“Tx”). Upper urinary tract pathology was excluded by computed urography (CTU), and urinary tract infection was ruled out by urine culture. The instillations began 4–6 weeks after the TURBT.
Electromotive force-assisted chemotherapy was applied with an EMDA device (Physionizer ® 30, manufactured by Physion®, Medolla, Italy). This device consists of a generator that emits an electrical current between a specialized 16 Fr catheter that works as an anode, and which introduces the MMC into the bladder (40 mg diluted in 50 mg of distilled water), and a cathode in the form of a patch located on the hypogastrium of the patient (Common Terminology Criteria for Adverse Events 2017). Sessions are carried out with a current of 20 mA for 30 min.
Conductive chemohyperthermia was administered with the Combat BRS System V2.0 (Combat Medical Ltd, Wheathampstead, UK). This is a dry, external device that heats 40 mg of MMC diluted in 50 mL of distilled water to 43 ± 0 0.5 ℃, which is then introduced into the bladder at 200 mL /min for 60 min.
The administration protocol is similar in both MMC groups. First, an induction of six weekly instillations was performed, followed by six monthly instillations as maintenance.
Patients in the third arm received an induction of six weekly instillations of BCG (50 mg of OncoCITE® diluted in 50 ml of normal saline) for 60 min, followed by maintenance therapy: three weekly instillations at months 3, 6, and 12.
Patient follow-up
Response to treatment was evaluated every 3 months by cystoscopy and urinary cytology. CTU was performed at months 6, 12, and 24, and annually thereafter, or when clinically indicated. TUR was performed when abnormalities were observed in the cystoscopy, or for suspicion of recurrence by imaging tests. When cytology was positive, biopsies of the bladder mucosa were taken.
Adverse events were assessed at each treatment and follow-up visit. Patients were asked about adverse events (AEs) and assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (Colombo et al. 2011). In case of grade 1 or 2 toxicity, symptomatic treatment was given, and instillation postponed. Treatment was discontinued if an allergy or a degree of toxicity > 3. Dose reduction was not allowed, only delaying treatment. The study ends when the patient withdraws informed consent or when an adverse event occurs that causes treatment to be delayed by more than 2 weeks. Patients who present a recurrence or progression are dropped from the study, but continue to be followed up.
Outcomes
The primary objective was to compare the 24-month recurrence-free rate between the three groups. The secondary objectives were the evaluation of the free rate of progression at 24 months, the time until recurrence and progression, and the toxicity of the treatments.
Patient rating
Recurrence: Appearance of a tumor of a lower grade or similar to the initial one, confirmed histologically.
Time to recurrence: Time from the first instillation to the next surgery in which recurrence was confirmed.
Progression: Appearance of muscle-invasive bladder cancer or CIS, confirmed histologically.
Time to progression: Time from the first instillation to the next surgery in which progression was confirmed.
Patients without progression or recurrence at the last follow-up test were censored. Patients lost to follow-up were censored on the last known day of survival.
Distribution and statistical analyses
All three therapies were available simultaneously. Each patient chose their allocation after a discussion with a group of clinicians who explained the evidence and emphasized the adverse effects. Therefore, it is a non-randomized study.
All data were collected prospectively, and retrospective analysis was performed.
Categorical and continuous variables were analyzed by performing the ANOVA Chi-square, Fisher exact, and Student t tests. The Kaplan–Meier method was used to assess time-to-event outcomes and curves were constructed for each study arm. Comparison was estimated by use of long-rank test. All tests were two-sided and a p value of < 0.05 was considered statistically significant.
Statistical analyses were performed with SPSS V23.0 (Armonk, NY: IBM Corp.)
Results
Patients
Initially, 190 patients agreed to participate in the study. Subsequently, six were excluded for presenting stage T2 disease in the R-TUR, and one withdrew before starting treatment. Finally, 183 patients were included and divided into 3 groups: HIVEC 61, EMDA 59, BCG 63. The baseline characteristics of the patients are comparable between the three groups (Table 1). In the three groups, being male, having primary tumors, and tumors smaller than 3 cm predominate.
Table 1.
Patient and tumor characteristics at baseline
| Variable | HIVEC | EMDA | BCG | p value |
|---|---|---|---|---|
| n = 61 | n = 59 | n = 63 | ||
| Gender, n (%) | 0.862 | |||
| Male | 47 (77%) | 47 (79.7%) | 51 (81%) | |
| Female | 14 (23%) | 12 (20.3%) | 12 (19%) | |
| Age, median (IQR) | 74 (65–80) | 71 (64–76) | 72 (66–78) | 0.391 |
| Tumor status, n (%) | 0.152 | |||
| Primary | 38 (62.3%) | 39 (66.1%) | 49 (77.8%) | |
| Recurrent | 15 (37.7%) | 20 (33.9%) | 14 (22.2%) | |
| Number of tumors, n (%) | 0.257 | |||
| Single | 26 (42.6%) | 28 (47.5%) | 36 (57.1%) | |
| Multiple | 35 (57.4%) | 31 (52.5%) | 27 (42.9%) | |
| Maximum tumor diameter, n (%) | 0.158 | |||
| < 3 cm | 46 (75.4%) | 35 (59.3%) | 44 (69.8%) | |
| ≥ 3 cm | 18 (24.6%) | 24 (40.7%) | 19 (31.7%) | |
| Tumor stage, n (%) | 0.358 | |||
| Ta | 37 (60.7%) | 37 (62.7%) | 32 (50.8%) | |
| T1 | 24 (39.3%) | 22 (37.3%) | 31 (49.2%) | |
| WHO Grade 2004/2016, n (%) | 0.458 | |||
| Low grade | 27 (44.3%) | 23 (39%) | 21 (33.3%) | |
| High grade | 34 (55.7%) | 36 (61%) | 42 (66.7%) | |
| Group of risk of EAU | 0.419 | |||
| Intermediate | 38 (62.3%) | 32 (54.2%) | 32 (50.8%) | |
| High | 23 (37.7%) | 27 (45.8%) | 31 (49.2%) | |
EAU European Association of Urology, SD Standard Deviation
Efficacy
The total mean follow-up of the patients was similar in the three groups, with a median of 28 months (IQR 13–38) for the HIVEC group, 21 months (IQR 11–34) for the EMDA group, and 28 (IQR 16- 35) for the BCG group (p > 0.05).
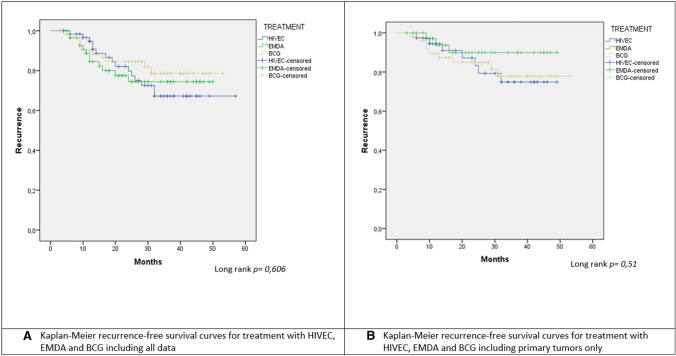
There were no differences in the recurrence-free rate at 24 months among the three treatments: HIVEC: 82.1%; EMDA: 80%; BCG: 84.6% (p > 0.05). In total, 15 recurrences (24.6%) occurred in the HIVEC group, 12 in the EMDA group (20.3%), and 11 in the BCG group (20.8%) with a median time to recurrence of 19.3 months for the HIVEC group, 12.5 for EMDA and 14 months for BCG (p > 0.05). Although significance was not reached when comparing the mean time to recurrence; the HIVEC group tends to present later recurrences compared to the other two groups.
The 24-month progression-free rate for the three groups was 95.6% for HIVEC, 98.3% for EMDA, and 92.9% for BCG (p = 0.263). Two progressions to CIS occurred in the HIVEC group at 12 and 19 months; one in the EMDA group progressed to CIS at 5 months, and five progressions occurred in the BCG group: three progressions to T2 and two progressions to higher tumor grade with a median time to progression of 15.6 months.
We also performed a Cox regression analysis in which we found that the applied treatment did not influence the recurrence prognosis after adjusting for the variables sex, age, previous recurrence, number of tumors, size, T stage, and grade (Table 2A).
Table 2.
Multivariate logistic regression analysis
| Haz.ratio | Std.err | Z | p | [95% conf. interval] | ||
|---|---|---|---|---|---|---|
| A. Including all data | ||||||
| HIVEC (ref) | – | – | – | – | – | – |
| EMDA | .8418224 | .3419603 | – 0.42 | 0.672 | .3797084 | 1.86634 |
| BCG | .909973 | .3806598 | – 0.23 | 0.822 | .4008243 | 2.06587 |
| Test of proportional-hazards assumption p = 0.3550 | ||||||
| B. Including primary tumors only | ||||||
| HIVEC (ref) | – | – | – | – | – | – |
| EMDA | .4276943 | .3061625 | – 1.19 | 0.235 | .1051491 | 1.739647 |
| BCG | 1.037203 | .5557003 | 0.07 | 0.946 | .3629255 | 2.96422 |
Test of proportional-hazards assumption p = 0.7180
Finally, we performed the same analyses including only the population with primary tumors, without finding significant differences in the recurrence-free rate between the treatment groups (long-rank p = 0.51) (Fig. 1B.) Nor were differences observed when performing the Cox regression analysis adjusted for the same variables previously described. (Table 2B).
Fig. 1.
A Kaplan–Meier recurrence-free survival curves for treatment with HIVEC, EMDA, and BCG including all data. B Kaplan–Meier recurrence-free survival curves for treatment with HIVEC, EMDA, and BCG including primary tumors only
Toxicity
Regarding the number of instillations, the QHT group received a mean of 9.9 (SD 2.5) instillations, the EMDA group received a mean of 8.8 (SD 3.5) instillations, and the BCG group received a mean of 12.7. (SD 3.2) instillations. There were significant differences between the mean number of instillations received by patients in the BCG group and the other two (p < 0.05), because the administration protocol of the mitomycin groups is different from the BCG group.
In general, there were no statistically significant differences among the three groups with respect to the degree of reported adverse events (p = 0.393). However, within the group of grade 1 adverse events, there were differences among treatment groups (p < 0.05). Bladder spasms prevailed in the HIVEC group, bladder spasms and skin burns in the EMDA group, and dysuria, urinary infection, and general malaise in the BCG group (Table 3).
Table 3.
Patient-reported adverse events
| Adverse events reported | HIVEC | EMDA | BCG | p |
|---|---|---|---|---|
| 0.393 | ||||
| None n, (%) | 41 (67.2%) | 33 (55.9%) | 28 (44.4%) | |
| Grade 1 n, (%) | 13 (23.2%) | 17 (28.8%) | 23 (36.5%) | |
| Dysuria, n | 0 | 3 | 6 | < 0.05 |
| Bladder spams, n | 12 | 5 | 2 | |
| Skin Burns, n | 0 | 5 | 0 | |
| Urinary tract infection, n | 0 | 2 | 6 | |
| Rash | 1 | 0 | 0 | |
| Nocturia | 0 | 1 | 0 | |
| Hematuria | 0 | 1 | 2 | |
| General malaise, n | 0 | 1 | 5 | |
| Fever | 0 | 0 | 2 | |
| Grade 2 n, (%) | 5 (8.2%) | 6 (10.2%) | 9 (14.3%) | |
| Dysuria | 0 | 1 | 3 | 0.126 |
| Urinary tract infection, n | 1 | 1 | 2 | |
| Skin burns, n | 0 | 2 | 0 | |
| Bladder spams, n | 2 | 2 | 0 | |
| Hematuria, n | 1 | 0 | 1 | |
| General malaise, n | 1 | 0 | 0 | |
| Fever, n | 0 | 0 | 3 | |
| Grade 3 n, (%) | 1 (1.6%) | 2 (3.4%) | 3 (4.8%) | |
| General malaise, n | 1 | 1 | 2 | 0.549 |
| Urinary tract infection, n | 0 | 1 | 1 | |
| Grade 4 n, (%) | 1 (1.6%) | 1 (1.7%) | 0 (0%) | |
| MMC allergy, n | 1 | 1 | 0 | |
| Grade 5 n, (%) | 0 (0%) | 0 (0%) | 0 (0%) |
During the treatment period, two patients in the chemohyperthermia group discontinued treatment due to adverse events, one experienced a grade 3 AE (general malaise) and the second a grade 4 AE (MMC allergy). In the EMDA group, eight patients discontinued treatment due to adverse events—three due to bladder spasms (grade 2), three due to urinary treat infection (one grade 3 patient, two grade 2 patients), one due to general malaise (grade 3) and one due to a severe adverse reaction due to a previously unknown drug allergy (grade 4). In the BCG group, there were six withdrawals from treatment as a result of adverse events. Two patients withdrew due to urinary infection (grade 2 and 3), three patients due to dysuria (grade 2), and one patient due to fever (grade 2).
At subsequent follow-up, two patients from the HIVEC group dropped out due to fear of COVID-19, three patients from the EMDA group and three from the BCG group died. All died from causes unrelated to their neoplastic disease or treatments.
Discussion
We present the first study comparing the application of MMC with hyperthermia versus MMC applied with EMDA and the administration of BCG as adjuvant treatment in patients with intermediate and high risk of NMIBC without CIS. Our results show that the three treatments have similar efficacy. There were no differences in the recurrence-free rate or progression at 24-month follow-up. From a safety point of view, there were no differences between the three groups with respect to the degree of reported adverse events, although there were differences in the predominant type of adverse effect. The importance of the results of our study is supported in the current context in which alternatives to BCG treatment are sought through the use of devices that enhance chemotherapeutic agents. However, although devices with different mechanisms of action have been developed, no study compares them with each other and with standard adjuvant treatment.
While this is the first study to compare MMC application with the COMBAT® and EMDA® devices versus BCG as adjunctive therapy, Colombo et al. (Stasi et al. 2003) in 2001 published a non-randomized trial in which MMC was applied with hyperthermia using Synergo® device or with EMDA, versus MMC alone as ablative treatment—demonstrating the superiority of the first two over the last.
Our results are in line with those published in studies supporting the use of these devices as adjuvant treatment. Di Stasi et al. (Stasi et al. 2003) published the results of a clinical trial in which 108 patients with NMIBC with CIS were allocated to receive treatment with passive MMMC, EMDA® or BCG, obtaining a RFR of 47.2% for the EMDA and BCG groups and 15% for the MMC group (p < 0.05) after 43 months of follow-up. Colombo et al. (2011) randomized 83 patients with intermediate- and high-risk NMIBC to receive treatment with MMC alone or boosted with RITE obtaining a better RFR for the study group (60 vs. 20%; p < 0.05). Subsequently, Arends et al. (2016) reported the results of their trial comparing hyperthermia treatment versus BCG in a population of 190 patients with intermediate- and high-risk BCG naïve NMIBC, also obtaining favorable results for the study group (RFR 81.8 vs. 64.8%; p < 0.05). Regarding the COMBAT device, Guerrero-Ramos et al. (2022) conducted a clinical trial in which they randomized 50 patients with high-risk NMIBC without CIS, obtaining a RFR of 86.3% at 24 months in the COMBAT® group and 71.8% in the BCG group (p > 0.05). In a single study, in which only the population with intermediate-risk NMIBC was selected, no differences were found between the application of MMC alone versus boosted with COMBAT with a 2-year disease-free survival rate similar between groups (61 vs. 60%) (Tan et al. 2022).
Regarding toxicity, in our study, as in previously published studies, most adverse effects are classified as mild (Plata et al. 2021; Colombo et al. 20001) with a predominance of local effects such as dysuria, bladder pain, or bladder spasms (Guerrero-Ramos et al. 2022; Stasi et al. 2003; Tan et al. 2022).
Being a prospective study with consecutive inclusion of patients, we were able to reduce the risks of information bias and underreporting of side effects. However, despite obtaining comparable groups, the lack of randomization is a limitation of our study.
Conclusion
In this study, no differences were observed in the recurrence-free or progression-free rate at 24 months among the adjuvant treatments with BCG and MMC applied with the EMDA or COMBAT device in patients with intermediate- and high-risk NMIBC and without CIS. For this reason, randomized studies would provide more evidence on the non-inferiority of MMC applied with the EMDA or COMBAT device compared to BCG in the treatment of patients with NMIBC.
Acknowledgements
This work was supported by the Hospital Clínico San Cecilio de Granada. We also acknowledge the Doctorate Program in Clinical Medicine and Public Health of the University of Granada, in which a thesis is being carried out regarding these devices
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Pareja Vilchez Manolo, Melgarejo Segura María Teresa, Morales Martínez Ana, and Yáñez Castillo Yaiza. The first draft of the manuscript was written by Arrabal Polo Miguel Angel and Arrabal Martín Miguel. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Ethical approval
The study obtained the approval of the Local Ethics Committee before experiment was started (Approved 27/7/2018. IRB number 0992-N-18).
Consent to participate
Written informed consent was obtained from the parents.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the manuscript and images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Miguel Ángel Arrabal Polo, Email: arrabalp29@gmail.com.
María Teresa Melgarejo Segura, Email: mtm.segura@gmail.com.
Yaiza Yáñez Castillo, Email: ymyc10@gmail.com.
Ana Morales Martínez, Email: anamorales891@hotmail.com.
Manuel Pareja Vílchez, Email: manuelparejavilchez@gmail.com.
Miguel Arrabal Martín, Email: arrabalm8@gmail.com.
References
- Arends TJH, Nativ O, Maffezzini M, de Cobelli O, Canepa G, Verweij F, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus Calmette-Guérin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol. 2016;69(6):1046–1052. doi: 10.1016/j.eururo.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65(1):69–76. doi: 10.1016/j.eururo.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Colombo R, Brausi M, Da Pozzo L, Salonia A, Montorsi F, Scattoni V, et al. Thermo-chemotherapy and electromotive drug administration of mitomycin C in superficial bladder cancer eradication a pilot study on marker lesion. Eur Urol. 2001;39(1):95–100. doi: 10.1159/000052419. [DOI] [PubMed] [Google Scholar]
- Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21(23):4270–4276. doi: 10.1200/JCO.2003.01.089. [DOI] [PubMed] [Google Scholar]
- Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC) BJU Int. 2011;107(6):912–918. doi: 10.1111/j.1464-410X.2010.09654.x. [DOI] [PubMed] [Google Scholar]
- Common Terminology Criteria for Adverse Events (CTCAE). 2017;155. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Di Stasi SM, Riedl C. Updates in intravesical electromotive drug administration of mitomycin-C for non-muscle invasive bladder cancer. World J Urol. 2009;27(3):325–330. doi: 10.1007/s00345-009-0389-x. [DOI] [PubMed] [Google Scholar]
- Di Stasi SM, Giannantoni A, Stephen RL, Capelli G, Navarra P, Massoud R, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. J Urol. 2003;170(3):777–782. doi: 10.1097/01.ju.0000080568.91703.18. [DOI] [PubMed] [Google Scholar]
- Guerrero-Ramos F, González-Padilla DA, González-Díaz A, de la Rosa-Kehrmann F, Rodríguez-Antolín A, Inman BA, et al. Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: results of the HIVEC-HR randomized clinical trial. World J Urol. 2022;40:999–1004. doi: 10.1007/s00345-022-03928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus calmette-guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63(3):462–472. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- Ojea A, Nogueira JL, Solsona E, Flores N, Gómez JMF, Molina JR, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus calmette-guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus Mitomycin C. Eur Urol. 2007;52(5):1398–1406. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Plata A, Guerrero-Ramos F, Garcia C, González-Díaz A, Gonzalez-Valcárcel I, de la Morena JM, et al. Long-term experience with hyperthermic chemotherapy (HIVEC) using Mitomycin-C in patients with non-muscle invasive bladder cancer in Spain. J Clin Med. 2021;10(21):5105. doi: 10.3390/jcm10215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racioppi M, Di Gianfrancesco L, Ragonese M, Palermo G, Sacco E, Bassi PF. ElectroMotive drug administration (EMDA) of Mitomycin C as first-line salvage therapy in high risk ‘BCG failure’ non muscle invasive bladder cancer: 3 years follow-up outcomes. BMC Cancer. 2018;18(1):1224. doi: 10.1186/s12885-018-5134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Kunath F, Coles B, Draeger DL, Krabbe LM, Dersch R, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD011935.pub2/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Wymer KM, Borah BJ, Saigal CS, Litwin MS, Packiam VT, et al. Cost-effectiveness of maintenance bacillus Calmette-Guérin for intermediate and high risk Nonmuscle invasive bladder cancer. J Urol. 2020 doi: 10.1097/JU.0000000000001023. [DOI] [PubMed] [Google Scholar]
- Sousa A, Piñeiro I, Rodríguez S, Aparici V, Monserrat V, Neira P, et al. Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate–high-risk non-muscle-invasive bladder cancer. Int J Hyperth. 2016;32(4):374–380. doi: 10.3109/02656736.2016.1142618. [DOI] [PubMed] [Google Scholar]
- Sylvester RJ, Rodríguez O, Hernández V, Turturica D, Bauerová L, Bruins HM, et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel. Eur Urol. 2021;79(4):480–488. doi: 10.1016/j.eururo.2020.12.033. [DOI] [PubMed] [Google Scholar]
- Tan WS, Kelly JD. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol. 2018;15(11):667–685. doi: 10.1038/s41585-018-0092-z. [DOI] [PubMed] [Google Scholar]
- Tan WS, Panchal A, Buckley L, Devall AJ, Loubière LS, Pope AM, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus Calmette-Guérin or institutional standard in patients with recurrence of non-muscle-invasive bladder cancer following induction or maintenance bacillus calmette-guérin therapy (HYMN): a phase III, open-label. Randomised Controlled Trial Eur Urol. 2019;75(1):63–71. doi: 10.1016/j.eururo.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Tan WS, Prendergast A, Ackerman C, Yogeswaran Y, Cresswell J, Mariappan P, et al. adjuvant intravesical chemohyperthermia versus passive chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer (HIVEC-II): a Phase 2, open-label. Randomised Controlled Trial Eur Urol. 2022;S0302–2838(22):02552. doi: 10.1016/j.eururo.2022.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.