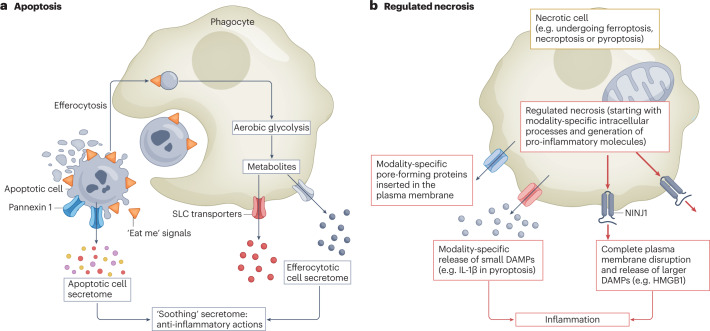
Fig. 2. Interaction between apoptosis, regulated necrosis and inflammation.
a, The process of apoptosis clears unwanted or damaged cells during development and under homeostatic conditions. Multiple steps within the process of apoptosis convey signals to dampen inflammation. Cells that are undergoing apoptosis display cell surface molecules (‘eat me’ signals) that promote their rapid engulfment (efferocytosis) and clearance by adjacent cells before the plasma membrane ruptures and the pro-inflammatory contents are released11. Additionally, caspase-mediated opening of pannexin 1 channels at the plasma membrane of the apoptotic cell facilitates the release of an apoptotic metabolite secretome that comprises AMP, GMP, creatine, spermidine, glycerol-3-phosphate, ATP, fumarate, succinate and other compounds that suppress inflammation in adjacent cells12. Furthermore, efferocytosis itself is associated with changes in phagocyte metabolism, including a switch to aerobic glycolysis and the release of glycolytic by-products such as lactate through solute carrier family (SLC) transporters to promote anti-inflammatory responses in adjacent cells13. Thus, overall the processes of apoptosis and efferocytosis are associated with a complex programme that promotes the ‘soothing’ of adjacent cells. b, By contrast, regulated necrosis can engage innate and adaptive immune responses at multiple steps through, for example, the lack of early engulfment, the generation of pro-inflammatory molecules (for example, IL-1β in pyroptosis) and the release of small damage-associated molecular patterns (DAMPs) through pore-forming proteins that are specific for each form of regulated necrosis. The final common pathway of these actions is disruption of the plasma membranes through the involvement of NINJ1, which allows the release of larger DAMPs.