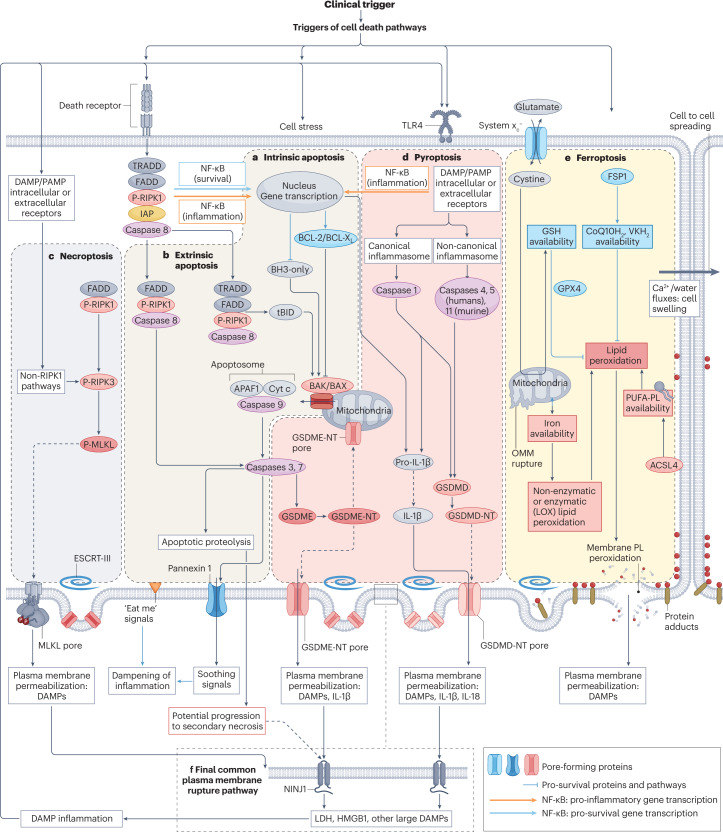
Fig. 3. Key molecular processes and interactions between cell death pathways.
Two main pathways for apoptosis are well characterized. a, In the intrinsic pathway, stressors or the lack of survival signals decrease the balance between pro-survival BCL-2 family proteins and pro-apoptotic BH3-only proteins, favouring the BAK/BAX-dependent mitochondrial outer membrane permeabilization, which results in the release of pro-apoptotic mitochondrial proteins, including cytochrome c, and leads to the formation of a multiprotein structure called the apoptosome that incorporates APAF1 and procaspase 9. This series of events results in activation of caspase 9 — an initiator caspase that subsequently activates executioner caspases such as caspase 3 and caspase 7 that contribute decisively to the dismantling of cell structures. b, In the extrinsic apoptosis pathway, activation of death receptors of the tumour necrosis factor receptor (TNFR) superfamily leads to activation of the initiator caspase 8, which in turn activates executioner caspases and may also process the BH3-only protein BID to tBID, which activates the mitochondrial apoptotic pathway. However, there are alternative outcomes for TNFR activation by TNF, depending on the recruitment of specific proteins to multiprotein complexes. The presence of inhibitor of apoptosis (IAP) proteins activates NF-κB to increase the transcription of anti-apoptotic proteins, although NF-κB can also induce a pro-inflammatory response. In absence of IAPs, caspase 8 is activated. Inhibition of caspase 8 may trigger necroptosis (see below). Apoptotic cells express “eat-me” signals on their surface, which promotes their rapid engulfment (efferocytosis) by adjacent cells. In addition, they secrete anti-inflammatory molecules (soothing signals). However, extensive apoptosis or decreased clearance of apoptotic cells may lead to secondary necrosis, in which the cell membrane is permeabilized through engagement of NINJ1 and damage-associated molecular patterns (DAMPs) released. c, Necroptosis. The core features of necroptosis are receptor-interacting serine/threonine-protein kinase 3 (RIPK3) activation by phosphorylation and the subsequent phosphorylation of mixed lineage kinase domain-like protein (MLKL). Phosphorylated MLKL oligomerizes to form pores that disrupt the plasma membrane, allowing the release of cell contents. Canonical RIPK3 activation is mediated by its interaction with the RIP homotypic interaction motif-containing proteins RIPK1, TIR domain-containing adapter molecule 1 (TICAM1) or Z-DNA-binding protein 1 (ZBP1). Binding of RIPK3 to RIPK1 occurs in response to activation of TNFRs, whereas binding of RIPK3 to TICAM1 is induced by activation of Toll-like receptors (TLRs), and binding to ZBP1 occurs in response to the presence of Z-RNA as a result of viral infection. Non-canonical RIPK3 activation occurs in response to activation of the cell membrane Na+/H+ exchanger 1 and increased intracellular pH (not shown). d, Pyroptosis. The key feature of pyroptosis is the enzymatic processing of gasdermins to amino-terminal (NT) fragments that oligomerize to assemble a plasma membrane pore, allowing the release of intracellular contents and DAMPs. The best-characterized model involves the NLRP3 (canonical) inflammasome-mediated activation of caspase 1 in macrophages, which processes pro-IL-1β to IL-1β, pro-IL-18 to IL-18 and gasdermin D (GSDMD) to GSDMD-NT. However, other inflammasomes and enzymes may process gasdermins in other cell systems and IL-1β may not be a major component of pyroptosis in cell types with low IL-1β gene expression. Additionally, gasdermins may permeabilize mitochondria, which, together with the potential for caspase 3 to cleave gasdermin E (GSDME) and caspase 8 to cleave GSDMD, provides links to apoptosis. e, Ferroptosis. The central event in ferroptosis is the peroxidation of plasma membrane phospholipids (PL) in an iron-dependent manner. The sensitivity of cells to ferroptosis will depend on iron availability (noting that mitochondria may serve to store iron), and on the capacity for enzymatic (for example, mediated by lipoxygenases (LOX)) or non-enzymatic lipid peroxidation as well as on the presence of cell defences against lipid peroxidation, including adequate glutathione (GSH) stores which are maintained through the entry of cystine through the system xc− cystine/glutamate antiporter. The enzyme glutathione peroxidase 4 (GPX4) requires GSH for its antioxidant function. Additional antioxidant systems include ferroptosis suppressor protein 1 (FSP1), which maintains vitamin K (VK) and coenzyme Q10 (CoQ10; also known as ubiquinone) in a reduced state (VKH2 and CoQ10H2, respectively). The main consequence of ferroptosis is peroxidation of membrane PL, shown as red dots in the figure, that results in membrane protein adducts and membrane rupture. Following some triggers, ferroptosis may spread from cell to cell in a wave-like pattern via a process that involves volume shifts and calcium fluxes, as has been described in kidney tubules. f, Shared features between the different cell death modalities include the ability of the repair machinery, endosomal sorting complexes required for transport III (ESCRT-III), to repair membranes and the need for NINJ1 in the final step of plasma membrane fragmentation, which leads to the release of the larger DAMPs and proteins. However, NINJ1 is not required for plasma membrane rupture during ferroptosis. Of note, although all molecular pathways in the figure are represented in the same cell to emphasize the interconnections between pathways, not all cell types have the intracellular machinery or microenvironment required for all forms of regulated cell death to proceed. For pyroptosis, the increased transcription, processing and release of IL-1β have been mainly characterized in macrophages, whereas epithelial cells have a more limited capacity to release this interleukin. ACSL, long-chain fatty acid–CoA ligase 4; OMM, outer mitochondrial membrane; PAMP, pathogen-associated molecular pattern; PUFA, polyunsaturated fatty acid; PUFA-PL, PUFA-containing phospholipids.