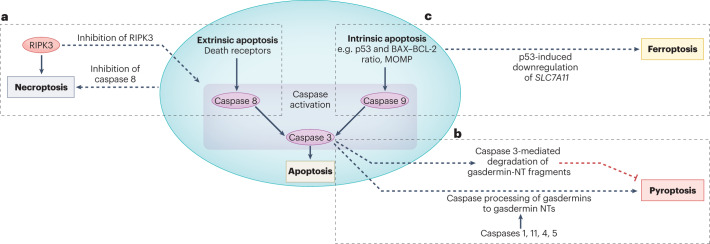
Fig. 4. Examples of interactions between apoptosis and regulated necrosis.
The molecular pathways that lead to activation of extrinsic or intrinsic apoptosis may also modulate or trigger different forms of regulated necrosis. Thus, interventions aimed at inhibiting apoptosis may trigger or modulate modalities of regulated necrosis, potentially affecting their safety and efficacy. a, Following the activation of death receptors, such as TNF receptor, inhibition of caspase 8 promotes necroptosis over apoptosis. Conversely, inhibitors of receptor-interacting serine/threonine-protein kinase 3 (RIPK)3 or certain kinase-dead RIPK3 mutants will prevent necroptosis but result in the assembly of multimeric protein complexes that activate caspase 8 and induce apoptotic cell death. b, Caspases are among the enzymes than can cleave gasdermins to generate amino-terminal (NT) fragments that oligomerize at the plasma membrane to form protein pores and trigger pyroptosis. In pyroptosis, canonical activation of the NLRP3 inflammasome results in activation of caspase 1, whereas non-canonical inflammasomes will activate murine caspase 11 (caspase 4 and caspase 5 in humans). In apoptosis, the executioner caspase 3 is activated by either caspase 8 (extrinsic apoptosis pathway) or caspase 9 (intrinsic apoptosis pathway). Caspases that are activated during apoptosis, such as caspase 8 and caspase 3 may also cleave gasdermins to gasdermin-NT fragments, as exemplified here for caspase 3. Additionally, caspase 3 may degrade NT fragments from gasdermins B and D, potentially protecting from pyroptosis. A more detailed representation of the interaction of caspases with gasdermins and gasdermin NTs is shown in Supplementary Fig. 4b. c, Upstream regulators of apoptosis may also modulate ferroptosis. As an example, the tumour-suppressive transcription factor p53 promotes apoptosis by upregulating mediators of apoptosis, such as BAX, and downregulating anti-apoptotic molecules such as BCL-2 (refs. 53,54), but also sensitizes cells to ferroptosis by downregulating SLC7A11, which encodes a subunit of the system xc− cystine–glutamate antiporter, and consequently decreasing cystine uptake55. MOMP, mitochondrial outer membrane permeabilization.