Figure 1.
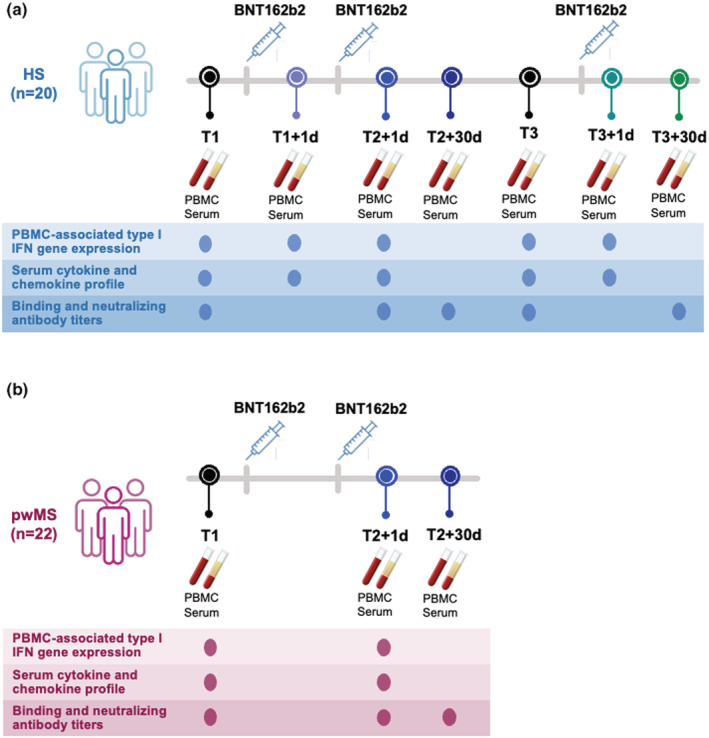
Scheme of enrolled healthy and Multiple Sclerosis vaccine recipients and collected samples. A schematic representation of the number of study participants and timeline of sample collection are depicted. At each time point, performed assays are reported. All enrolled subjects did not undergo SARS‐CoV‐2 infection during the study period. (a) Healthy subjects (HS, n = 20) were enrolled before and after undergoing administration of the 3 doses of the Pfizer‐BioNTech BNT162b2 mRNA‐based anti‐COVID‐19 vaccine. Peripheral blood mononuclear cells (PBMC) and serum samples from longitudinal peripheral blood withdrawals were collected at the following time points: immediately before the first dose (T1), 1 day and 30 days after the first vaccine dose (T1 + 1d and T1 + 30d, respectively), 1 day and 30 days after the second vaccine dose (T2 + 1d and T2 + 30d), as well as right before and 1 day and 30 days after the third boosting dose (T3, T3 + 1d and T3 + 30d). (b) People with relapsing remitting Multiple Sclerosis (pwMS, n = 22) were enrolled before and after undergoing administration of the first 2 doses of the Pfizer‐BioNTech BNT162b2 mRNA‐based anti‐COVID‐19 vaccine. Peripheral blood mononuclear cells (PBMC) and serum samples were longitudinally collected before first dose (T1) and after 1 day or 30 days from second dose of Pfizer‐BioNTech BNT162b2 mRNA‐based anti‐COVID‐19 vaccine (T2 + 1d and T2 + 30d, respectively).
