Figure 3. The role of liquid-liquid phase separation in cellular quality control.
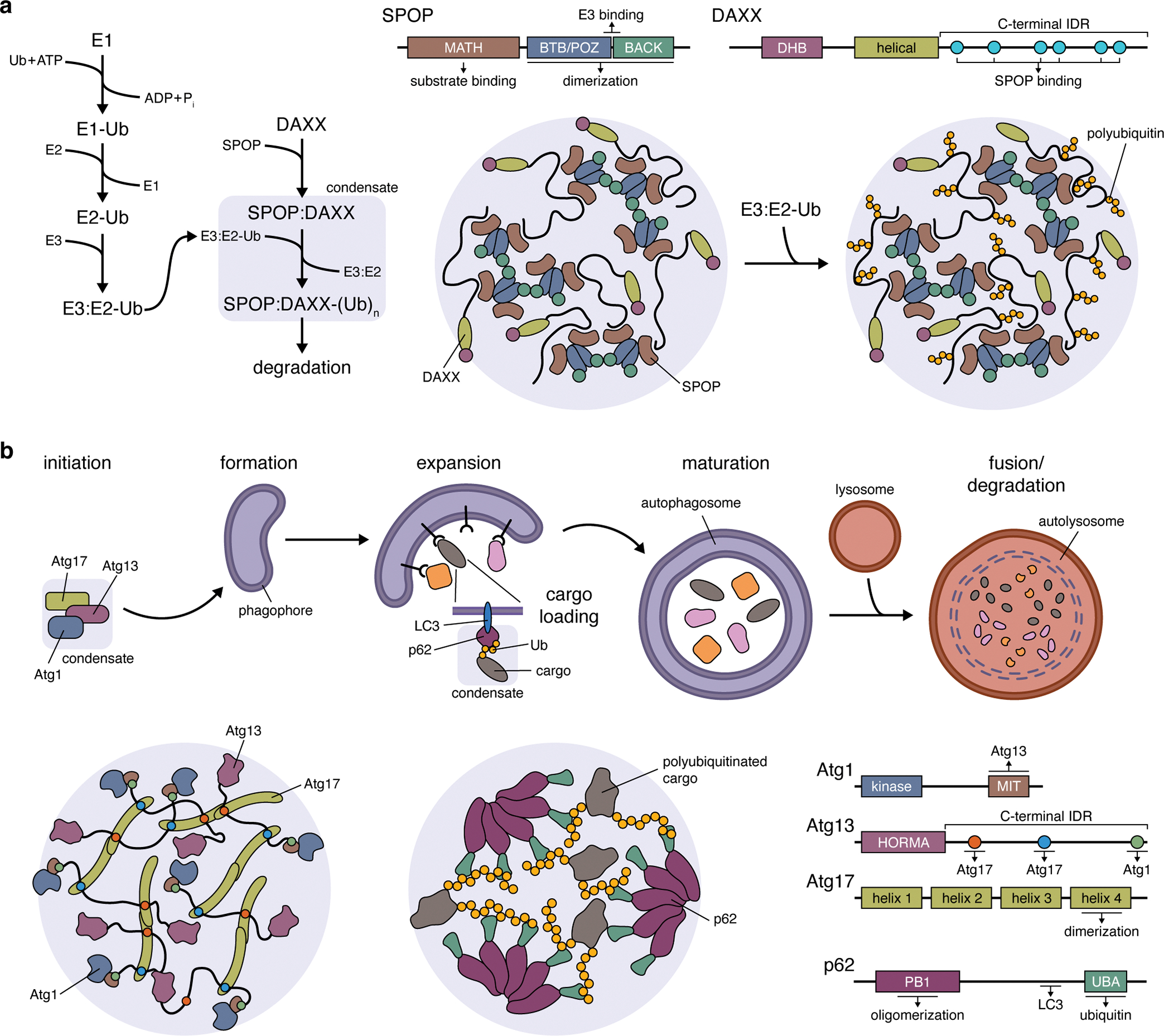
(a) The formation of biomolecular condensates between speckle-type POZ protein (SPOP) and its target death-domain-associated protein (DAXX). Left: Pathway schematic highlighting the role of SPOP:DAXX condensates in facilitating DAXX polyubiquitination. Right: LLPS of SPOP and DAXX is driven by the formation of SPOP dimers through its BR-C/ttk/bab (BTB)/Pox virus and zinc finger (POZ) domain, interactions between SPOP dimers mediated by their BTB and C-terminal Kelch (BACK) domains, as well as interactions between the long, C-terminal IDR of DAXX with multiple copies of the N-terminal meprin and TRAF homology (MATH) domain of SPOP. The BTB/POZ-BACK domain junction in SPOP also binds E3 ubiquitin ligases, recruiting them into condensates (omitted from cartoon) to promote DAXX polyubiquitination. Ub, ubiquitin; (Ub)n, polyubiquitin; E1, Ub-activating enzyme; E2, Ub-conjugating enzyme; E3, Ub-ligase. (b) Top: Simplified schematic of the autophagy pathway, highlighting the involvement of Atg1, Atg13, and Atg17 condensates in autophagy initiation and the role of p62 condensates in cargo loading during phagophore expansion. Bottom: Atg1:Atg13:Atg17 condensates (left panel) are scaffolded by Atg13 and Atg17, with the C-terminal IDR of Atg13 containing a pair of binding sites that crosslink Atg17 dimers. Atg1 is recruited as a client protein via its C-terminal microtubule-interacting and transport (MIT) domain, which binds Atg13. Bottom: LLPS of p62 condensates (middle panel) requires the formation of p62 oligomers, mediated by an N-terminal Phox and Bem1p (PB1) domain, whose C-terminal ubiquitin-associated (UBA) domains then participate in multivalent interactions with the ubiquitin chains on polyubiquitinated substrate proteins.
