Figure 2.
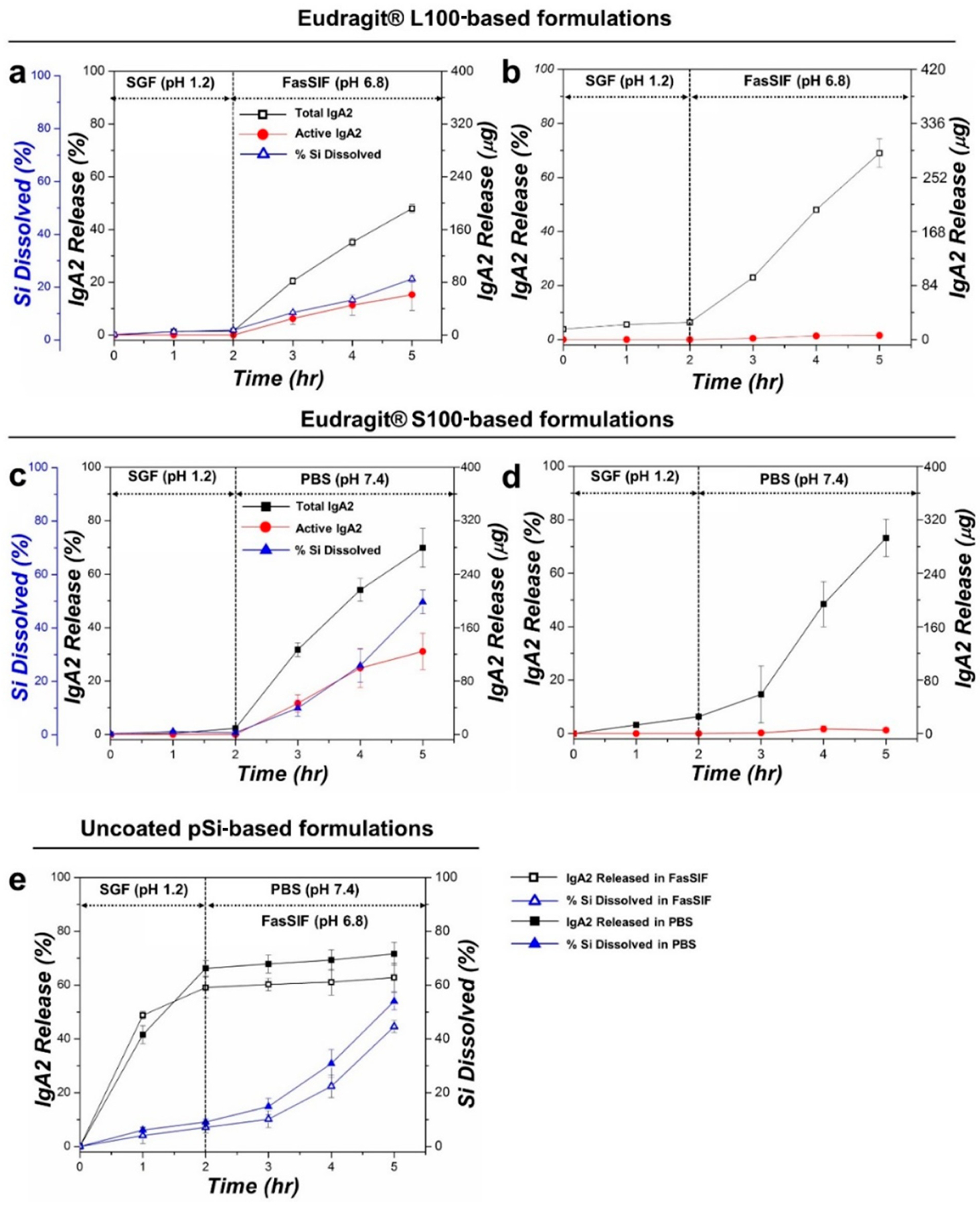
Release of IgA2 from enteric polymer-coated pSi particle formulations and from controls, comparing total protein (Total IgA2) with protein activity (Active IgA2) as a function of time: (a) Eudragit L100-coated pSi particles, (b) Eudragit L100 polymer control (IgA2 loaded into polymer only), (c) Eudragit S100-coated pSi particles, (d) Eudragit S100 polymer control, and (e) uncoated pSi particle control. For the compositions that used the pSi carrier, the percent of dissolved Si measured in the elution solution is also given. These temporal release profiles were obtained following an elution protocol mimicking oral delivery to either the small intestine or to the colon, involving a 2 h incubation in simulated gastric fluid (SGF, pH 1.2) followed by either fasting-state simulated intestinal fluid (FasSIF, pH 6.8) or phosphate buffered saline (PBS, pH 7.4) for 3 h. The FasSIF condition was designed to simulate delivery to the small intestine, whereas the PBS condition simulated delivery to the colon. The temperature throughout the experiments was maintained at 37 °C. The total protein was measured by BCA assay, the total active IgA2 antibody was measured by ELISA (for Eudragit–pSi formulations, activity was further confirmed with a stringent HIV neutralization assay), and the % Si present in eluents was measured by molybdenum blue assay.
