Figure 4.
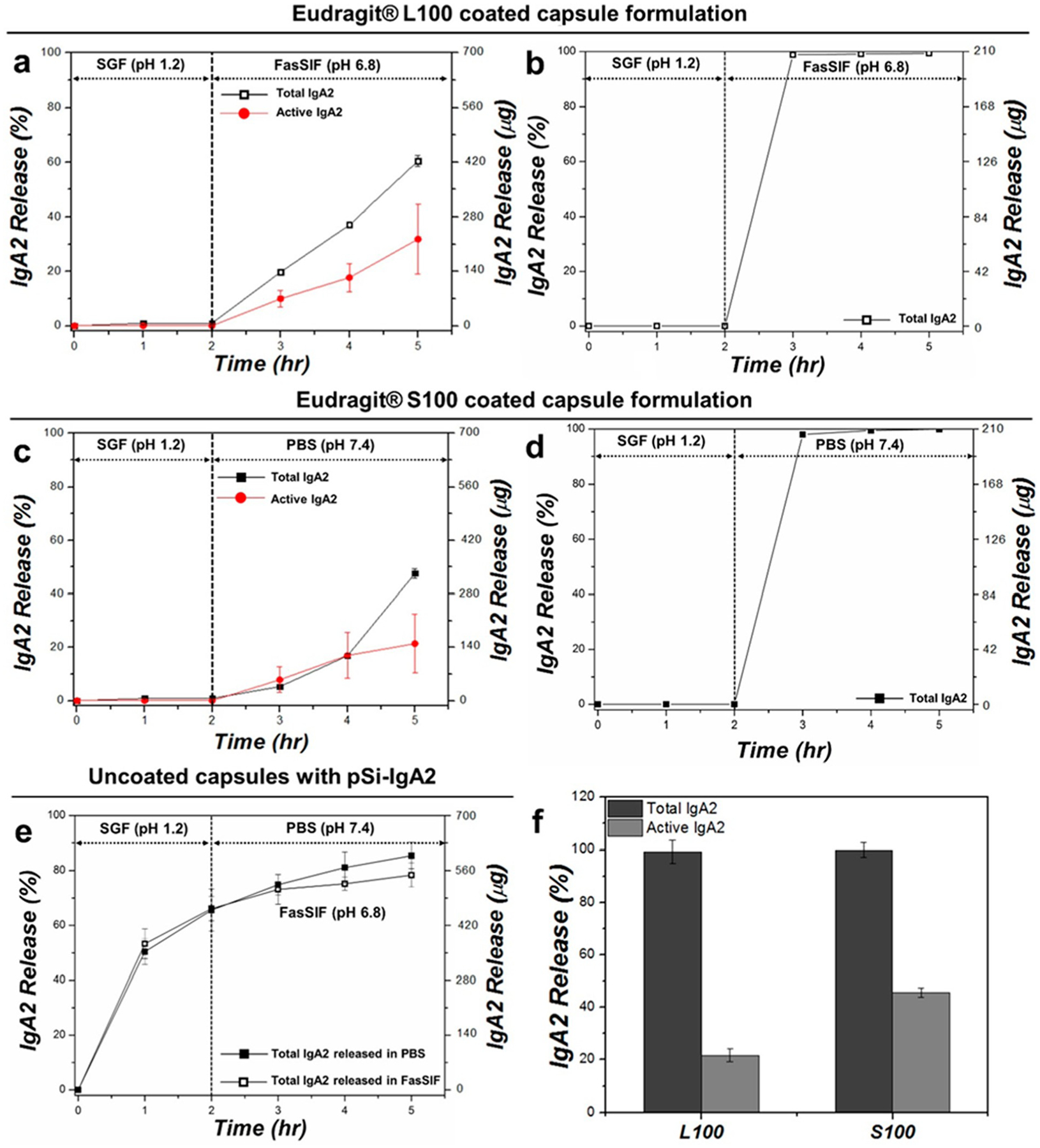
Release of IgA2 from capsule-packed formulations and from controls, comparing total protein (Total IgA2) with protein activity (Active IgA2) as a function of time: (a) total and active IgA2 released from Eudragit L100-coated capsule packed with IgA2-loaded pSi particles, (b) total IgA2 released from Eudragit L100-coated capsule packed with vacuum-dried IgA2 without pSi particles, (c) total and active IgA2 released from Eudragit S100-coated capsule packed with IgA2-loaded pSi particles, (d) total IgA2 released from Eudragit S100-coated capsule packed with vacuum-dried IgA2 without pSi particles, (e) total IgA2 released from uncoated capsule packed with IgA2-loaded pSi particles in SGF for 2 h followed by release in either FasSIF (pH 6.8; open black squares) or PBS (pH 7.4; closed black squares), and (f) total (BCA assay) and active (ELISA assay) IgA2 released at 3 h time point from (b) and (d) (i.e., IgA2-packed Eudragit polymer-coated capsules without pSi). Since negligible quantities of IgA2 were released in SGF during the first 2 h of incubation and approximately 99% of the IgA2 packed in the capsule was released during the following 3 h time point, activity was determined at this point only.
