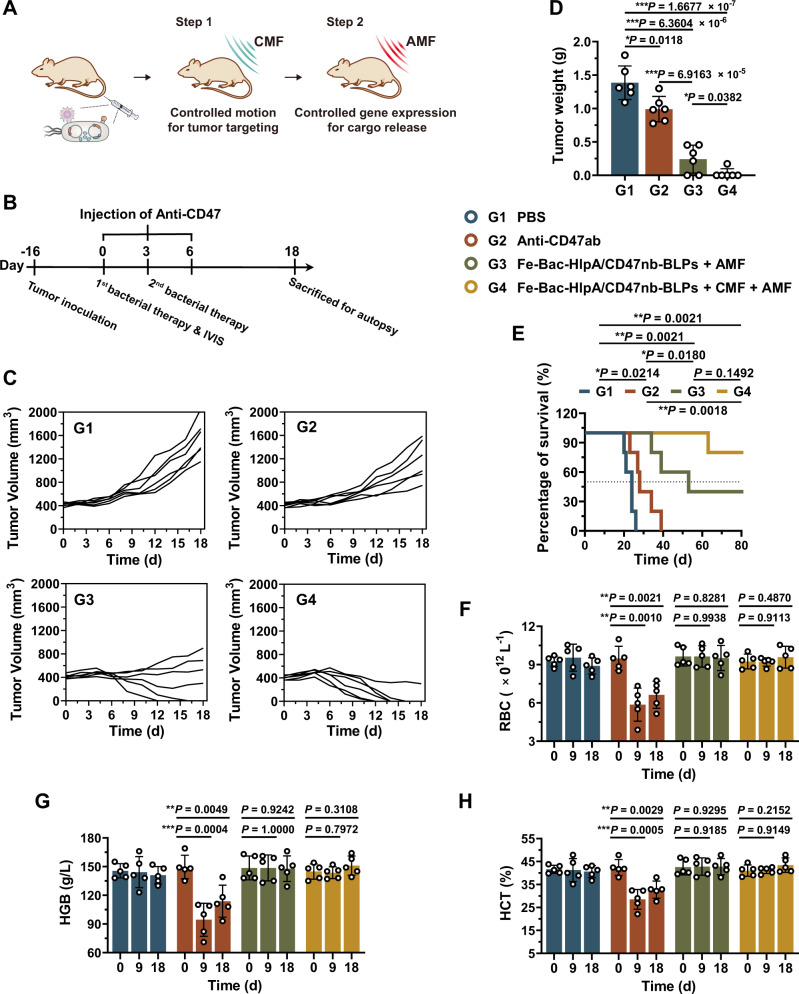
Fig. 10. The combination of CMF-controlled motion and AMF-controlled gene expression could achieve excellent therapeutic efficacy with minimal side effects.
A Illustration of the sequential combination of CMF-controlled motion for tumor targeting and AMF-controlled gene expression for cargo release to achieve excellent therapeutic effects of AMF-Bac. After i.v. administration, AMF-Bac exhibits biased and directional motion towards tumors upon the guidance of CMF (Step 1). Once reaching tumors, AMF-Bac initiates the gene expression of BLPs for bacterial lysis to release the CD47nb cargo (Step 2). B Scheme and grouping of in vivo antitumor therapy. BALB/c mice were subcutaneously inoculated with CT-26-luc cells (2 × 106 cells/mouse) in the right hind limb on day −16 to allow the average tumor volume of ~420 mm3 at day 0, followed by different treatments. For G2, Anti-CD47ab (20 mg/kg) were i.p. injected on days 0, 3, and 6, respectively. For G3, mice were i.v. injected with the Fe-Bac-HlpA/CD47nb-BLPs (5 × 106 CFU) on days 0 and 3, followed by AMF treatment (310 kHz and 23.8 kA/m) for 80 min at 24 h after injection. For G4, mice were i.v. injected with the Fe-Bac-HlpA/CD47nb-BLPs on days 0 and 3, and the NdFeB magnet was placed close to the tumor for 4 h after injection, enabling CMF to guide the bacterial motion swimming towards tumors, followed by the same AMF treatment for 80 min at 24 h after injection. C Change in tumor volume of the subcutaneous CT-26-luc (n = 6). D Tumor weight of the subcutaneous CT-26-luc xenografts measured on day 18 (n = 6 mice). E Survival curves of mice from the indicated groups for 80 days (n = 5 mice). F–H The changes in RBC (E), HGB (F), and HCT (G) during therapy (n = 5 mice). The data (D, E–H) are shown as the mean ± SD. Statistical analysis was performed by a two-tailed unpaired t test. Survival significance was analyzed by the log-rank test. *P < 0.05; **P < 0.01; ***P < 0.001. Source data are provided as a Source Data file.