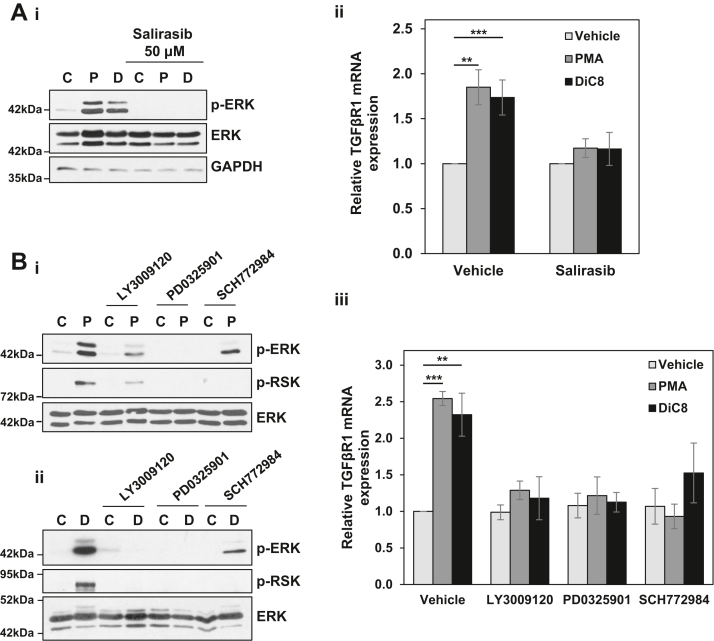
Figure 3.
TGFβR1 is regulated by PKCα-ERK signaling. A, serum-starved IEC-18 cells were pretreated with 50 μM Salirasib prior to treatment with vehicle (C), 100 nM PMA (P), or 20 μg/ml DiC8 (D) for 2 h. Expression and phosphorylation of the indicated proteins were assessed by Western blotting (i), and TGFβR1 mRNA levels (normalized to 18S rRNA) were determined by RT-qPCR (ii). B, as in A except that cells in full serum medium were pretreated with 1 μM LY3009120, 10 μM PD0325901, or 1 μM SCH772984. Note that the reduction in ERK phosphorylation in the presence of SCH772984 is an “on-target” effect of this inhibitor that has been attributed to its ability to induce conformational changes in ERK that impair its interaction with MEK (e.g., (80)). Data in Ai, Bi, and Bii are representative of at least three independent experiments. Data in Aii and Biii are the average ± SEM of at least three independent experiments. ∗∗p < 0.01. ∗∗∗p < 0.001. DiC8, 1,2-dioctanoyl-sn-glycerol; ERK, extracellular signal-regulated kinase 1/2; IEC-18, intestinal crypt-like cells; MEK, mitogen-activated protein kinase kinase 1/2; PKCα, protein kinase C α; PMA, phorbol 12-myristate 13-acetate; TGFβR1, transforming growth factor-β receptor 1.