Abstract
Acute low back pain (LBP) stands as a leading cause of activity limitation and work absenteeism, and its associated healthcare expenditures are expected to become substantial when acute LBP develops into a chronic and even refractory condition. Therefore, early intervention is crucial to prevent progression to chronic pain, for which the management is particularly challenging and the most effective pharmacological therapy is still controversial. Current guideline treatment recommendations vary and are mostly driven by expertise with opinion differing across different interventions. Thus, it is difficult to formulate evidence-based guidance when the relatively few randomized clinical trials have explored the diagnosis and management of LBP while employing different selection criteria, statistical analyses, and outcome measurements. This narrative review aims to provide a critical appraisal of current acute LBP management by discussing the unmet needs and areas of improvement from bench-to-bedside, and proposes multimodal analgesia as the way forward to attain an effective and prolonged pain relief and functional recovery in patients with acute LBP.
Keywords: Low back pain, Guidelines, Gaps, Evidence based, Acute pain, Analgesics, Multimodal analgesia, Fixed dose combination
Key Summary Points
| Why carry out this study? |
| Acute low back pain (LBP) stands as a leading cause of disability, and its associated healthcare expenditures become substantial when acute LBP develops into a chronic and even refractory condition. |
| Current guideline treatment recommendations vary and are mostly driven by expertise with opinion differing across different interventions. |
| It is difficult to formulate evidence-based guidance when the relatively few randomized clinical trials have explored the diagnosis and management of LBP while employing different selection criteria, statistical analyses, and outcome measurements. |
| What was learned from the study? |
| Early intervention is crucial to prevent progression of acute to chronic pain, for which management is particularly challenging and the most effective pharmacological therapy is still controversial. |
| It is paramount to better align practice with the evidence and to place greater efforts to facilitate the implementation of interventions able to ease the patient management burden, both from the physician’s and patient’s perspective. |
| Multimodal analgesia stands as the way forward to attain an effective and prolonged pain relief and functional recovery in patients with acute LBP. |
Introduction
Low back pain (LBP) is a widespread musculoskeletal condition [1], and the global burden of disability associated with this condition has been increasing, particularly within the working-age population, with approximately 70% of years lost through disability in working-aged people and among women compared with men [2, 3]. Thus, LBP occurrence is associated with early retirement, work absenteeism, and loss of productivity presenteeism while being at work [4]. In patients with LBP, pain severity and disability are longitudinally associated to health-related quality of life (HRQoL), and healthcare costs [5, 6]. Overall, strategies to mitigate LBP burden are needed, and the recognition that it is one of the most pressing public health priorities is required [6]. Several barriers have hindered an effective acute LBP management so far. First, it is characterized by a complex etiology (mechanical, neurological, and systemic causes) and underlying pain mechanisms (nociceptive, neuropathic). Second, it is associated with a significant degree of heterogeneity and intrinsic variability. Third, a high rate of recurrence has been documented for acute LBP within 1 year after the first acute episode that may evolve in chronic and disabling pain [7, 8], depending on risk factors for chronicity such as obesity, smoking, severe disability, and depression/anxiety [9]. Identifying the source of pain is still challenging for most clinicians, especially in the primary care setting where patients seek first help in most cases [1]. Practitioners are mostly dealing with patients within a biomedical framework despite the opportunities provided by the biopsychosocial model of LBP, including the conceptualization of LPB etiology and prognosis, as well as the development and testing of many interventions [10]. Overall, there are substantial evidence-to-practice gaps, and a clear need of promoting a better translation of pain knowledge to clinical practice as recently advocated by IASP with the launch of the 2022 Global Year advocacy campaign [11]. The multifactorial nature of LBP supports a multimodal treatment approach by combining analgesic agents with different modes of action [12]. Mounting evidence suggests that a multimodal analgesic approach to LBP patients can provide effective and adequate pain control, along with a greater improvement of patients’ satisfaction with therapy [13]. This narrative review aims to provide a critical appraisal of current acute LBP management by discussing the unmet needs and areas of improvement from bench-to-bedside, and proposes multimodal analgesia as the way forward to attain an effective and prolonged pain relief and functional recovery in patients with acute LBP.
Selection of Evidence
Papers considered for the present narrative review were retrieved via a keyword-based query of multiple databases including PubMed, Google Scholar, and Cochrane library database (e.g., “low-back pain” AND “acute pain” AND “multimodal therapy” AND “multimodal analgesia”), without limitations in terms of publication date. The search was last updated in July 2022 and was limited to papers in English. Papers were selected for inclusion according to their relevance for the topic, as judged by the Authors. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
LBP: When a Complex Pathophysiology and a Heterogeneous Patient Profiles Hinder an Appropriate Patient Care
Acute LBP covers a range of frequently overlapping different types of pain including nociceptive, neuropathic, or nonspecific pain. Vulnerability of the elements encompassing the lumbar spine (e.g., soft tissue, vertebrae, zygapophyseal and sacroiliac joints, intervertebral discs, and neurovascular structures) to different stressors can lead to LBP. Given the low specificity of imaging and diagnostic injections, the diagnosis of this condition continues to be controversial [14]. Clinicians can reliably differentiate acute and persistent mechanical LBP from back pain resulting from a specific cause [15] via a full assessment of key signs and symptoms along with evaluation of red flags. Failure to recognize serious causes early on results in delayed testing and treatment, and may increase patient morbidity and mortality [15]. Red flags can be caused by tumors, infections, fractures, and neurological damage, and if they are present, the patient should be evaluated by the appropriate specialist(s) to get the necessary treatment as part of the overall treatment plan [16] (Table 1). In addition, three categories of acute LBP—the so-called “diagnostic triage”—can be identified, namely, serious spinal pathology, nerve root pain/radicular pain, and nonspecific low back pain [17]. Of note, it is paramount that an accurate diagnosis of pain generators is determined before starting any treatment. Following identification of red flags, excluding the possibility of neuropathic LBP is often the first step in clinical practice.
Table 1.
| Red flags unrelated to specific disease | Red flags endorsed for specific disease |
|---|---|
| Age of onset less than 20 years or more than 55 years | Malignancy |
| Recent history of violent trauma | History of malignancies/cancer |
| Constant progressive, nonmechanical pain (no relief with bed rest) | Unexplained/unintentional weight loss |
| Thoracic (or abdominal) pain | Pain |
| Past medical history of malignant tumor and of major/significant trauma | Age over 50 years |
| Prolonged use of corticosteroids | Fracture |
| Drug abuse, immunosuppression, human immunodeficiency virus | History of major/significant trauma |
| Systemically unwell | Systemic use of steroids |
| Unexplained/ unintentional weight loss | Infection |
| Widespread sensory deficit (in lower limbs) | Fever ≥ 38 °C |
| Fever ≥ 38 °C | Use of corticosteroids or immunosuppressant therapy |
| Cauda equina syndrome | |
| Bladder dysfunction | |
| Sphincter disturbance |
Clinicians managing patients with LBP often encounter difficulty in differentiating between nociceptive/mechanical and neuropathic pain and selecting the most appropriate pain management strategies, that is, those directed at peripheral and central processes. Such diagnostic uncertainty is associated with limited response to treatment and poor patient outcomes, including unnecessary suffering [18]. The variable LBP disease course and the limited knowledge of pain and disability trajectories also contribute to the currently inadequate provision of LBP care.
It has been increasingly understood that patients with LBP are not experiencing episodes of unrelated occurrences, but rather are suffering from a long-lived condition with a fluctuating course with a trajectory of ongoing or fluctuating pain of low-to-moderate intensity [19, 20]. Importantly, few patients may quickly get well while others may suffer from persistent, severe acute LBP, experience a recurrence within 12 months after recovery, and easily progress to chronic LBP when presenting comorbidities, mental health issues, and poor general health [8, 21, 22]. The most frequent factors promoting recurrence and chronicity in LBP have been investigated by a systematic review and encompass a history of LBP (at least more than two previous episodes), low level of job satisfaction, awkward posture, and longer time sitting [23]. Once chronic, LBP is particularly problematic to manage; thus, preventing the transition from acute to chronic LBP is important. It has been reported that between 2% and 48% of patients with acute LBP in primary care settings transition to chronic LBP; of note, these data are in line with a reported overall 32% transition rate to chronic LBP at 6 months [9, 24]. Accordingly, the prevention of progression to chronic pattern of pain is also a pressing issue in LBP management. Increased risk of chronic pain has been associated with a history of compensation for a spinal condition, receipt of work-related sickness payments, or litigation about compensation [25]. However, very recent evidence from a systematic review identified as the most frequently observed risk factors for chronic LBP greater pain intensity, obese status, difficult working positions, and depression. Finally, general anxiety, smoking, and mainly physical work can act as predictors of chronicity [26]. Although attaining a full recovery after LBP can be an ambitious goal, advances in our understanding of the predictors of lack of recovery (such as levels of baseline pain intensity, pain catastrophizing, and depressive symptoms) from acute LBP may inform therapeutic decisions [27]. Finally, from a clinical standpoint, it has been documented that although trajectories of pain and disability may develop in parallel, their psychological predictors may differ. For example, if eradicating pain is not achievable, addressing the psychosocial barriers underlying the development and maintenance of disability may be a goal in pain rehabilitation [27].
LBP Practice Guidelines: Current Gaps, Limitations, and Areas of Improvement
To optimize clinical practice and sustainable access to healthcare resources, reducing variability of care and implementing evidence-based diagnostic and therapeutic approaches are paramount. To this end, clinical practice guidelines (CPGs) can act as a pillar in the promotion of an improved LBP quality of care [28–30]. In 1987, the Quebec Task Force issued the first LBP CPG [31]. Since then, several multidisciplinary LBP guidelines, mostly created by an expert panel through consensus, have emerged, as well as a wide range of treatment options for back pain and ever-growing published evidence [32]. Such an overwhelming volume of evidence, often conflicting and of variable quality (Table 2), is currently hindering the implementation of guideline’ recommendations in routine settings. As a result, adherence to guideline-recommended treatments is largely variable [33–35] and more than one in five patients with LBP receive inadequate LBP care [36]. Accordingly, modest patient treatment satisfaction has emerged [37], with even insufficient provision of care being reported in patients with comorbidities [38]. Furthermore, if the scope of acute LBP guidelines would have been the prevention of chronic pain development and of the persistence of LBP-associated disability, recent data underscore current guidelines’ failure to meet such important goals, as more than one in five adults in the USA experiences chronic pain, with about 20.5 million (40.9%) reported being bothered “a lot” by back pain [39]. Globally, the years lived with disability (YLDs) of LBP were found to have increased by 52.7% from 1990 to 2017, with Western Europe displaying the greatest value of LBP YLDs [3].
Table 2.
Overview of clinical practice guidelines for acute LBP management. Elaborated from [35, 45, 46, 52, 53]
| Clinical guideline | Aim of the guideline | Clinical premise | Recommended intervention for acute LBP | Areas of inconclusive evidence | Ref |
|---|---|---|---|---|---|
| A Joint Clinical Practice Guideline from the American College of Physicians and the American Pain Society (2007) | To present the available evidence for evaluation and management of acute and chronic LBP in primary care settings | Clinicians should inform all patients of the generally favorable prognosis of acute LBP with or without sciatica, including a high likelihood for substantial improvement in the first month | Clinicians should provide patients with evidence-based information on LBP regarding their expected course, advise patients to remain active, and provide information about effective self-care options. Clinicians should consider the use of medications with proven benefits in conjunction with back care information and self-care. For most patients, first-line medication options are paracetamol or nonsteroidal antiinflammatory drugs (NSAIDs) | There is insufficient evidence to guide specific recommendations on the timing of or indications for referral, and expertise in management of LBP varies substantially among clinicians from different disciplines (including primary care providers) | Chou et al. Ann Intern Med. 2007;147:478–491 |
| Canadian practice guideline on primary care management of LBP (2015) | To increase the use of evidence-informed conservative approaches to the prevention, assessment, diagnosis, and treatment in primary care patients with LBP; to promote appropriate specialist referrals and use of diagnostic tests in patients with LBP; to encourage patients to engage in appropriate self-care activities | Practitioners should emphasize that acute low back pain is nearly always benign and generally resolves within 1–6 weeks. Physicians are encouraged to reinforce that pain typically resolves in a few weeks without intervention | Superficial heat (application of heating pads or heated blankets) is recommended for the short-term relief of acute LBP. Prescribe medication, if necessary, for pain relief preferably to be taken at regular intervals. First choice paracetamol, second choice NSAIDs |
Inconsistencies are found in the recommendation regarding herbal treatments Despite the publication demonstrating no benefit of paracetamol over placebo for LBP in primary care [56], paracetamol is recommended in acute and chronic LBP. Canadian guidelines advocate the use of tricyclic antidepressants (TCAs) and paracetamol. For many of these, the evidence base is either limited or not promising. Finally, Canadian guidelines offer detail on suggested care pathways, though these are largely determined by expertise rather than evidence |
|
| NICE Guideline on Low Back Pain and Sciatica NG59 (2016) | To improve people’s quality of life by promoting the most effective forms of care for LBP and sciatica | This guideline advocates education towards an “expected” course of LBP, in which the probability of a rapid improvement in symptoms is high, potentially to reduce the risk of fear/catastrophizing and to moderate expectations | NICE only recommends considering the use of NSAIDs, and if NSAIDs are ineffective, contraindicated, or not tolerated, then consider a weak opioid, with or without paracetamol for acute LBP where an NSAID could not be used |
The duration of symptoms was not specified. The recommended combination weak opioid/paracetamol for acute LBP is based on very limited evidence Across all the interventions reviewed by the NICE group, no intervention was considered to have strong enough evidence to warrant a clear “offer” recommendation. NICE guidelines offer detail on suggested care pathways, though these are largely determined by expertise rather than evidence |
National Guideline Centre (UK) “Low back pain and sciatica in over 16s” |
| A Clinical Practice Guideline from the American College of Physicians (2017) | To provide treatment guidance based on the efficacy, comparative effectiveness, and safety of noninvasive pharmacologic and nonpharmacologic treatments for acute (< 4 weeks), subacute (4 to 12 weeks), and chronic (> 12 weeks) LBP in primary care | Most patients with acute LBP improve over time regardless of treatment. Clinicians should inform all patients of the generally favorable prognosis of acute LBP with or without sciatica, including a high likelihood for substantial improvement in the first month | Clinicians and patients should select nonpharmacologic treatment with superficial heat (moderate-quality evidence), massage, acupuncture, or spinal manipulation (low-quality evidence). If pharmacologic treatment is desired, clinicians and patients should select NSAIDs or skeletal muscle relaxants (moderate-quality evidence) | Most RCTs enrolled a mixture of patients with acute, subacute, and chronic LBP, so it is difficult to extrapolate the benefits of treatment compared with its duration. Evidence on patient-important outcomes, such as disability or return to work, is largely unavailable | Qaseem et al. Ann Intern Med. 2017;166:514–530 |
| Clinical practice guidelines for the management of nonspecific low back pain in primary care (2018) | Clinical practice guidelines provide evidence-based recommendations to assist decision-making about health interventions | Most guidelines recommend reassuring the patient that LBP is not a serious illness regardless of the duration of symptoms or reassuring patients with acute LBP of the favorable prognosis | Most guidelines recommend advice to maintain normal activities for patients with acute LBP, and some guidelines recommend the same advice for patients with any duration of symptoms | Acute LBP is invariably defined as less than 4, 6, and 12 weeks. Discrepancies in the recommendations for the use of paracetamol, muscle relaxants, and herbal medicines. Most guidelines recommend the use of weak opioids for short periods if NSAIDs are contraindicated or not effective for patients with acute LBP, despite an absence of relevant clinical trials | Oliveira et al. European Spine Journal 2018; 27:2791–2803 |
Concerns on methodological limitations affecting the quality of guidelines have been previously raised, with early CPGs appraisals suggesting a generally poor quality of LBP CPGs, even though recently improved, and their limited applicability [40, 41]. Analyzing major LBP guidelines [42–45], several issues emerged, including uncertain value of the available interventions for LBP, inconsistency in clinical efficacy of the tested pharmacological approaches, and a wide variability in the range of pharmacological and interventional options recommended across guidelines. The latter issue may stem from the observation that guideline recommendations are driven by expertise in which opinions differ across different interventions [32]. A critical appraisal of the most recent CPGs for LBP interventions by means of the Appraisal of Guidelines Research and Evaluation (AGREE) II instrument, the gold standard for critical appraisal of guidelines, has been published recently [46]. Methodological limitations influencing the quality of CPGs were emphasized, including a very limited participation of patients and their advocates. Similarly, a very recent appraisal confirmed that CPGs varied in quality, with most being characterized by the lowest score in stakeholder involvement, rigor of development, and applicability [47].
To advance LBP patient care and support clinicians in management decisions, an evidence-based guidance is of utmost relevance. However, CGP recommendations have favored management approaches such as the “wait and see” approach that appears inadequate to effectively tackle the LBP burden as it is built on the erroneous assumption that most people with acute LBP will get well without any issue [7, 48]. Surprisingly, most guidelines (10 out of 14; 71%) for the management of nonspecific LBP in primary care recommend reassuring the patient that LBP is not a serious illness and that it may have a favorable prognosis (Table 2) [49, 50]. Current CPGs have supported, so far, the erroneous concept of “an expected course of LBP” that basically ignores the natural history of LBP and the well-documented trajectories of pain and disability. Although this stepped care approach seems promising given the shortage of resources available in most healthcare systems, a delayed intervention is particularly detrimental in patients at high risk of chronicity or in those suffering from comorbidities such as depression, which is a well-known correlate of chronic pain [51]. Of note, postponing adequate treatment may promote rather than prevent the transition from acute into subacute and chronic LBP [48].
Diagnostic workup with red flags and therapy recommendations for patients with LBP also vary across CPGs [52]. Although different red flags are present in LBP guidelines, there is no consensus between guidelines for which red flags to endorse and a marked variability in precise definitions of the red flags (e.g., “trauma,” “severe trauma,” “major trauma”). Overall, a core set of red flags ideally endorsed by all guidelines is largely awaited [52].
Although the use of nonsteroidal antiinflammatory drugs (NSAIDs) for patients with acute and chronic LBP is recommended while considering the risk of adverse events (e.g., renal, cardiovascular, and gastrointestinal), one in two CPGs still recommend in favor of paracetamol, despite no benefit of paracetamol over placebo for LBP in primary care being reported so far [32, 53]. Moreover, most guidelines recommend the use of weak opioids for short periods if NSAIDs are contraindicated or not effective for patients with acute LBP, despite an absence of relevant clinical trials and the potential increased harms for patients with nonspecific LBP [49].
Overall, there is a glaring demand for additional high-quality clinical evidence, possibly built upon a rigorous clinical trial design, an evidence-based medication choice, and broader inclusion criteria acknowledging both the heterogeneity and variability of LBP. In addition, clinical trials should also include, among the measured endpoints, not only the differences in pain intensity but also variation in pain severity, pain-related distress, and interference in daily activities, as well as improvement in functional disability [54, 55]. Meaningful tools such as the Roland–Morris Disability Questionnaire (RMDQ) can be of help in clinical settings, particularly in the follow-up period, to evaluate patients’ response and restoration of well-being. To this end, clinical trials should investigate how to increase the likelihood that patients will achieve outcomes that matter the most for them. Useful insights have been provided by the identification of core outcome domains for clinical trials in nonspecific LBP, namely “physical functioning,” “pain intensity,” “health-related quality of life,”and “number of deaths” [56]. Furthermore, it is desirable to gather evidence from head-to-head comparisons of newly marketed drugs with well-established treatment options. Such findings may help identifying first-line combination pharmacotherapy to guide through a rational approach in medical treatment of patients with LBP. This would finally create evidence-based guidelines rather than consensus-based guidelines, and potentially make it easier to implement in clinical routine settings [57]. Table 3 illustrates the gaps, limitations, and areas of improvement of currently available LBP CPGs.
Table 3.
Current gaps and areas of improvement in acute LBP management clinical practice guidelines. Elaborated from data in [3, 10, 35, 43, 49, 51]
| Current CPS gaps | Areas of improvement |
|---|---|
| CPGs are mostly consensus-based rather than evidence-based | Build high-quality clinical evidence upon a rigorous clinical trial design and evidence-based medication choice |
| CPGs are based on the assumption that LBP is short lived, benign, and effectively addressed by a stepped care approach | Gather evidence from studies exploring both pain and disability trajectories in patients with LBP, as well as identifying the factors predicting recurrence and chronicity |
| CPGs are characterized by a limited applicability and implementation in routine settings and a wide variability in the recommended pharmacological and interventional options | Gather evidence from head-to-head comparisons of newly released drugs with older agents to improve appropriateness of pharmacotherapy in clinical practice |
| CPGs provide conflicting evidence and of variable quality, and acknowledge limited participation of patients and their advocates | Design high-quality clinical evidence that investigate how to increase patients biopsychosocial benefits, submitting them to active questionnaires |
CPG clinical practice guideline, LBP low back pain
The Role of Multimodal Therapy in LBP Management
Multimodal therapy approaches are emerging as promising strategies to enhance clinical outcomes for patients with several diseases, including diabetes [58], obesity [59], rheumatic diseases [60], cancer [61], thrombotic diseases [62], and pain [13]. Regarding the latter, the objectives of multimodal therapy are to lower pain intensity and drug-related adverse events, to speed up recovery, and to facilitate rehabilitation. Ideally, multimodal therapy should restore patients’ functionality, ameliorate QoL, and prevent progression of acute to chronic pain [63]. The biopsychosocial model acknowledges that LBP derives from a dynamic cross-talk between social, psychological, and biological factors that can both predispose to and result from injury [64]; therefore, these factors should be taken into account when an interdisciplinary treatment plan is designed [14]. As the factors affecting the intensity and duration of acute LBP vary significantly, multimodal therapy stands as the most logical approach [48]. Therefore, pain relief can be achievable by targeting different sites of the nociceptive pathway and by managing the plethora of pain-related conditions, as well as pain correlates (e.g., depression, sleep abnormalities) through pharmacologic and nonpharmacologic modalities.
In one study, multimodal therapy (4 h per day for 20 days, consisting of medical training therapy, cognitive-behavioral therapy, physiotherapy, and patient education) was evaluated in a primary care setting and offered meaningful reduction in pain intensity, interference with daily living, depressive mood, and QoL [65]. It has also been reported to ease the recovery of physical functioning and subsequently the return to work-related activities [66]. The effectiveness of an inpatient follow-up after multimodal therapy in 155 patients with chronic LBP has also been evaluated [67]. Multimodal therapy improvement in terms of pain intensity, depression, anxiety, and well-being were significant after a 3-month follow-up. Of note, patients seemed to benefit more from attending multimodal therapy in an earlier stage of healthcare [68]. These findings further support the notion that early intervention is important in patients with acute LBP to prevent progression to chronic pain [69, 70].
Finally, providing high value care in LBP should mean placing greater attention on patient-reported outcomes and acknowledging the impact of patient satisfaction on treatment outcomes. It has been suggested that multimodal therapy aims to increase patient satisfaction in patients with acutely exacerbated chronic pain [71, 72]. A retrospective analysis evaluating multimodal treatment in 375 patients with chronic pain-related rheumatic diseases (111 of which reported LBP) supported this data [60]. Of note, after implementing multimodal therapy, a significant improvement of mental (mood) status was observed despite high levels of pain reported on admission in the study population; this improvement was also described in patients with LBP [60].
One key component of multimodal therapy is pharmacological treatment that is mostly geared toward analgesia and symptom management. Pharmacological treatments for the management of patients with LBP generally encompass paracetamol and NSAIDs as first-line treatment options, along with opioids, tricyclic antidepressants (TCAs), and anticonvulsants, the use of which depends on the type of LBP and patient history [38, 73]. However, evidence supporting the efficacy of paracetamol [53, 74] is insufficient for drawing firm conclusions, as acetaminophen was found not effective in reducing acute LBP [75] nor able to affect the time of recovery compared with placebo on a regular or as-needed dosing regimen [53]. In addition, no difference between paracetamol and placebo was documented in pain and disability at 1 week (immediate term); 2, 4, and 12 weeks (short term); or on QoL, function, global impression of recovery, and sleep quality [76]. Finally, conflicting results about the use of several NSAIDs in LBP have been reported [77]. Nevertheless, a patient-centered approach, acknowledging the patient’s other comorbidities, medications, and previously trialed treatments should guide treatment decisions.
Along with therapeutic interventions, interventional pain management modalities could be a useful component in multimodal treatment of LBP [70]. The most common include epidural steroid injections (ESI), radiofrequency ablation (RFA) of facet or sacroiliac joint innervation, intradiscal and vertebral augmentation procedures, and intrathecal drug delivery with implantable pump [78, 79]. Although ESI can be of help for short-term management of subacute/chronic LBP, no long-term effect on pain or surgical rates have been documented. Nevertheless, ESIs may often be used as a panacea for LBP, despite data showing that they are most effective for specific structural etiologies [80]. In patients who experienced the failure of other pain therapies, the use of implantable drug-delivery systems was associated with disability reduction and significant improvement of QoL and patient satisfaction with this therapy [81]. Overall, considering both the improvement of pain intensity in at least the short and medium terms, and the equivocal results in terms of functional improvement [79], further studies are required to fully support interventional pain procedures’ role in LBP management [82].
Multimodal Analgesia: the Way Forward
It has been suggested that LBP management should address the different patterns of pain trajectories (continuous pain along with acute flares) that characterize it [83], acting on the multiple pain generator mechanisms (either mechanical or neuropathic) to lower the risk of recurrence and, consequently, that of chronicity [38]. A recent Delphi study suggested that physicians would favor multidisciplinary-multimodal approaches to achieve the objectives of LBP management, thereby shifting towards treating LBP as a biopsychosocial issue that requires management in-kind [38]. Compared with monomodal analgesia, multimodal analgesia offers several advantages including greater analgesia, shorter hospitalization times, and improved recovery and function in postoperative and osteoarthritis [84]. Therefore, multimodal analgesia has been included in the current international guideline recommendations for both postoperative and osteoarthritis pain [85, 86].
When two or more analgesic medications are combined (either in free or fixed formulations) for pain relief, it allows for lower doses of each drug to be administered, thus limiting the risk of adverse drug effects with the maximum benefit. Of note, advantages of fixed-dose combination (FDC) products that may ease the patients’ management burden, include dosing convenience, reduction of pill burden, the potential for greater patient adherence, and, in the case of FDC products involving an opioid and a nonopioid agent, opioid-sparing effects and fewer side effects due to the reduced doses of each single substance [87]. Over time, multimodal analgesia has become more standard to manage pain as effectively as possible, also reducing opioid exposure [88] without sacrificing patient comfort or impeding rehabilitation [89]. This is relevant if one considers that opioids remain a common drug of choice for acute LBP in the emergency department (ED) [90] and their use in ED has been associated with an increased length of stay [91]. Another reported advantage of multimodal analgesia is the possible reduction in acute pain transition to chronic pain [92]. Such approach should be preferred in patients suffering from acute LBP whose risk of chronicity is worrisome and hinders patient functional recovery, thus further impairing patients’ QoL.
While waiting for novel agents, a major aim in current pain research is to use the existing drugs in a better way. Therefore, an effective analgesic FDC can be developed by combining a COX inhibitor with an opioid, whose clinical efficacy and tolerability profiles have been well documented. Therefore, clinicians should be aware that not all COX inhibitors are equally valuable as component of multimodal analgesia or equally as effective at providing the antiinflammatory and analgesic benefits with less untoward effects, mostly gastrointestinal (GI) and cardiovascular (CV). As per GI and CV toxicity, NSAIDs differ in terms of opioid-sparing effect [93]. Among NSAIDs, dexketoprofen provides a significant reduction in opioid use (36–50%), which is much greater than that attained upon treatment with diclofenac, ketorolac, and ibuprofen [93]. Celecoxib, a NSAID that acts primarily via inhibition of cyclooxygenase-2, has recently been combined in the novel co-crystal form of tramadol-celecoxib (CTC) 200 mg BID. As an urgent need for pain therapies to be effective and tolerated, in the context of multimodal analgesia, CTC has been developed for the management of acute moderate-to-severe pain. Unfortunately, in the latest randomized, double-blind, phase 3 STARDOM2 trial—in acute moderate-to–severe pain after abdominal hysterectomy—CTC was not superior to tramadol alone, failing to meet the primary endpoint [94].
Importantly, opioids also differ in terms of cardio–pulmonary tolerability, GI discomfort, and somnolence. Tramadol offers an alternative to other opioids as its two complementary synergistic actions, i.e., agonism to opioid receptor and inhibition of serotonin and norepinephrine re-uptake, enhance its pain relief effects and improve its tolerability profile. Unlike other weak opioids, tramadol has no relevant effects on CV and pulmonary parameters, and its administration is associated with less constipation and opioid-induced bowel dysfunction, along with a low addiction rate [95, 96].
Combinations of oral analgesics including tramadol were investigated with the twice-daily fixed combination of 75 mg tramadol/650 mg paracetamol (DDS-06C) in the treatment of moderate-to-severe acute LBP [97]. Although it did not include an active treatment arm as a comparator, in this study, the superior analgesic efficacy of DDS-06C versus placebo was confirmed for the primary efficacy endpoint. The relatively high response observed in the placebo group is consistent with the well-characterized “placebo response” observed in other pain studies [98, 99].
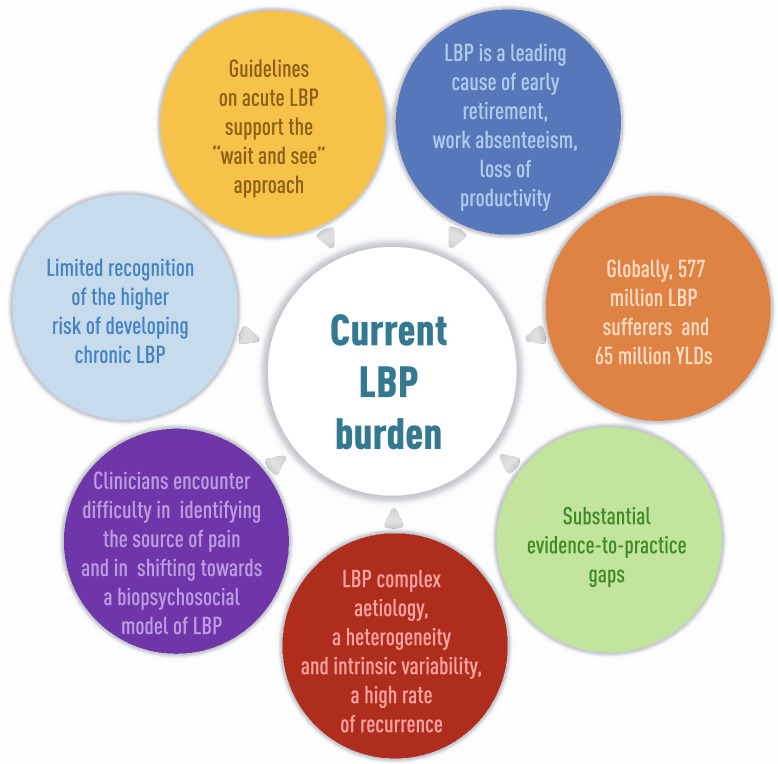
The fixed dose combination tramadol/dexketoprofen (TRAM/DKP) holds great promise for multimodal pain management. Of note, the rapid onset of analgesic effect of DKP, with its endpoint antiinflammatory activity associated with the sustained (mean duration: 8.1 h) action of TRAM, makes this combination a valuable tool to achieve multimodal analgesia [100–102]. Owing to its central analgesic effect, peripheral analgesic action, and antiinflammatory activity [83], TRAM/DKP may contribute to pain relief in acute exacerbations of LBP [103]. Recent observational studies in LBP patients showed that TRAM/DKP can be a valuable and effective option [104, 105]. However, such studies were single-center retrospective clinical trials with relatively small sample sizes (less than 100 patients each) and excluded patients with history of chronic LBP. As outlined in Table 3, there is a clear need to build high-quality clinical evidence to support effective acute LBP management. To this end, a multicenter, randomized, double-blind, double-dummy parallel group, placebo, and active controlled study (DANTE Study) is currently ongoing to prospectively assess the efficacy of TRAM/DKP in moderate-to-severe acute LBP with or without radiculopathy (EU register EudraCT number: 2019-003,656-37) [106]. Overall, the DANTE study aims to address some of the areas of improvement listed in Table 3, thus providing substantial advancement in the routine management of patients with acute LBP, thereby easing the considerable burden associated with such a disabling condition (Fig. 1).
Fig. 1.
Current LBP burden. Graphical elaboration of data in [3, 4, 10, 11, 15, 42, 51]. LBP low back pain, YLDs years lived with disability
Discussion
Worldwide, LBP ranks as the leading contributor to disease disability, and a recent World Health Organization (WHO) report has confirmed that LBP is the primary cause of disability in 160 countries [107]. Therefore, early intervention is pivotal in patients with acute LBP to prevent progression to chronic pain. Despite the available acute LBP treatment options, most of them lack a high level of evidence [32, 49, 50]. Current guideline treatment recommendations, being consensus-based, are mostly driven by expertise with opinion differing across different interventions. However, it is difficult to formulate evidence-based guidance when relatively few randomized clinical trials have investigated the diagnosis and management of LBP, and these have employed different selection criteria, statistical analyses, and outcome measurements. Therefore, further studies addressing the areas of improvement listed in Table 3 are urgently needed. Also, the existing guidance provided physicians with limited support to identify both the etiology of pain and the underlying pain mechanisms, and subsequently to guarantee the most appropriate therapeutic regimen for the specific patient. Importantly, patient education is recommended in treatment guidelines as a part of a multimodal approach to improve self-efficacy and coping strategies [49, 108]. Therefore, it is imperative for healthcare professionals to involve patients with LBP in the care process and have access to up-to-date, evidence-based information to assist clinicians in treatment decision-making. In this context, physicians’ education should be promoted as it is directly related to better patient outcomes, favoring patient responses to physicians' actions, thus leading to reductions in healthcare utilization [109]. Of note, clinicians should shift away more from the biomedical framework alone toward combining it with a biopsychosocial model, being aware of the potential negative implications of addressing only pain severity and ignoring what matters the most for patients, namely disability, functional impairment, and QoL [64]. Finally, pursuing a value-based care in LBP means working as integrated practice units centered around the patient’s clinical condition. Therefore, to increase awareness on the importance of communication among all the specialists the patient encounters along his/her disease journey should be a priority for all the scientific societies engaged in pain management. The words “low back pain” yield almost 44,000 results on PubMed, suggesting ever-expanding understanding of back pain and the associated psychological and social risk factors, as well as genetics factors. However, as recalled by many, this high volume of evidence represents a paradox as it has failed to translate into a clinical practice able to provide patients with LBP with the care they deserve [64, 110]. It is paramount to better align practice with the evidence, and to place greater efforts to facilitate the implementation of interventions able to ease the patient management burden, both from the physician’s and patient’s perspective. This means working towards a redesign of clinical pathways and patient journey, during which the patient will not face avoidable steps before appropriate care is given. To this end, easy to apply guidelines and practical tools useful in different care settings can be of help.
Our narrative review has provided a critical appraisal of CPGs that can be of help in evaluating strategies to manage pain in this major health issue setting, especially when the HCP could face a possible gap or unmet needs in guidelines. Hopefully, the unmet needs in LBP management highlighted here, and the promising role of multimodal analgesia described, could stimulate researchers to produce new evidence that can help in improving CPGs, with a consequent improvement in LBP management.
Conclusions
LBP represents one of the most difficult challenges for the healthcare professionals coping with pain patients. From the perspective of healthcare professionals, its epidemiology is difficult to accept. In its acute manifestation it must be treated rapidly and as well as possible, considering that it may become responsible for a transformation of pain from acute to chronic. Notwithstanding that, its incidence and prevalence are increasing, as well as the number of chronic low back pain patients is increasing. Generally, only poor-quality LBP CPGs are currently available, and many therapies have been suggested to physicians. Overall, there is a glaring demand for additional high-quality clinical evidence, possibly built upon a rigorous clinical trial design, with an evidence-based medication choice and broader inclusion criteria acknowledging both the heterogeneity and variability of LBP. A multispecialist and multimodal approach for management is a universally accepted concept. Inside this, multimodal pharmacologic therapy remains a cornerstone for acute LBP treatment. This must be as simple and efficacious as possible. The fixed-dose combinations of NSAIDs and weak opioids seem the most appealing multimodal pharmacological therapies available for these patients. Of these, the combination of dexketoprofen and tramadol, has excellent potential as therapy, already proven as very efficacious in other acute moderate-to-severe pain conditions.
Acknowledgements
Funding
The study and rapid service fee has been realized thanks to an unconditioned grant of the Menarini International Operations Luxembourg SA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in drafting the initial manuscript was provided by Dr. Chiara Degirolamo, Medical Writer, on behalf of Content Ed Net. Support for this assistance was funded by Menarini group. Moreover, the authors are grateful to the Paolo Procacci Foundation for its support in the publishing process.
Disclosures
The authors are consultants of a few companies, for scientific support. Magdi Hanna, Serge Perrot, Giustino Varrassi have nothing to disclose in relation to this publication.
Compliance with Ethics Guidelines
This study analyses previously published studies and does not require any approval by Ethics Committees.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Camilloni A, Nati G, Maggiolini P, et al. Chronic non-cancer pain in primary care: an Italian cross-sectional study. Signa Vitae. 2021;17(2):54–62. [Google Scholar]
- 2.Paterniani A, Sperati F, Esposito G, et al. Quality of life and disability of chronic non-cancer pain in adult patients attending pain clinics: a prospective, multicenter, observational study. Appl Nurs Res. 2020;56:151332. doi: 10.1016/j.apnr.2020.151332. [DOI] [PubMed] [Google Scholar]
- 3.Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 2020;8(6):299. doi: 10.21037/atm.2020.02.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grabovac I, Dorner TE. Association between low back pain and various everyday performances: activities of daily living, ability to work and sexual function. Wien Klin Wochenschr. 2019;131(21–22):541–549. doi: 10.1007/s00508-019-01542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutubuki EN, Beljon Y, Maas ET, et al. The longitudinal relationships between pain severity and disability versus health-related quality of life and costs among chronic low back pain patients. Qual Life Res. 2020;29(1):275–287. doi: 10.1007/s11136-019-02302-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchbinder R, van Tulder M, Öberg B, et al. Low back pain: a call for action. Lancet. 2018;391:2384–2388. doi: 10.1016/S0140-6736(18)30488-4. [DOI] [PubMed] [Google Scholar]
- 7.Linton SJ, Nicholas M, Shaw W. Why wait to address high-risk cases of acute low back pain? A comparison of stepped, stratified, and matched care. Pain. 2018;159:2437–2441. doi: 10.1097/j.pain.0000000000001308. [DOI] [PubMed] [Google Scholar]
- 8.Machado GC, Maher GC, Ferreira PH, Latimer J, Koes BW. Can recurrence after an acute episode of low back pain be predicted? Phys Ther. 2017;97:889–895. doi: 10.1093/ptj/pzx067. [DOI] [PubMed] [Google Scholar]
- 9.Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. 2021;4:e2037371. doi: 10.1001/jamanetworkopen.2020.37371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincus T, Kent P, Bronfort G, Loisel P, Pransky G, Hartvigsen J. Twenty-five years with the biopsychosocial model of low back pain-is it time to celebrate? A report from the twelfth international forum for primary care research on low back pain. Spine (Phila Pa 1976). 2013;38:2118–23. doi: 10.1097/BRS.0b013e3182a8c5d6. [DOI] [PubMed] [Google Scholar]
- 11.2022 Global Year advocacy campaign. Available at:https://www.iasp-pain.org/advocacy/global-year/translating-pain-knowledge-to-practice/ (Accessed August 2, 2022).
- 12.Müller-Schwefe G, Morlion B, Ahlbeck K, et al. Treatment for chronic low back pain: the focus should change to multimodal management that reflects the underlying pain mechanisms. Curr Med Res Opin. 2017;33:1199–1210. doi: 10.1080/03007995.2017.1298521. [DOI] [PubMed] [Google Scholar]
- 13.Paladini A, Varrassi G. Multimodal pharmacological analgesia in pain management. In “Pain Management”, IntechOpen. 2020. 10.5772/intechopen.93620.
- 14.Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021;398:78–92. doi: 10.1016/S0140-6736(21)00733-9. [DOI] [PubMed] [Google Scholar]
- 15.DePalma MG. Red flags of low back pain. JAAPA. 2020;33:8–11. doi: 10.1097/01.JAA.0000684112.91641.4c. [DOI] [PubMed] [Google Scholar]
- 16.Perrot S, Montero Matamala A, Hanna M, Varrassi G. The patient-centered approach in rheumatologic painful diseases: a narrative review. Cureus. 2022;14:e22244. doi: 10.7759/cureus.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Tulder M, Becker A, Bekkering T, et al. Chapter 3 European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J 2006;15:S169–S191. [DOI] [PMC free article] [PubMed]
- 18.Spahr N, Hodkinson D, Jolly K, et al. Distinguishing between nociceptive and neuropathic components in chronic low back pain using behavioural evaluation and sensory examination. Musculoskelet Sci Pract. 2017;27:40–48. doi: 10.1016/j.msksp.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn KM, Hestbaek L, Cassidy JD. Low back pain across the life course. Best Pract Res Clin Rheumatol. 2013;27:591–600. doi: 10.1016/j.berh.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Kongsted A, Kent P, Axen I, Downie AS, Dunn KM. What have we learned from ten years of trajectory research in low back pain? BMC Musculoskelet Disord. 2016;17:220. doi: 10.1186/s12891-016-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrot S, Allaert FA, Concas V, Laroche F. "When will I recover?" A national survey on patients' and physicians' expectations concerning the recovery time for acute back pain. Eur Spine J. 2009;18:419–429. doi: 10.1007/s00586-008-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva T, Mills K, Brown BT, et al. Recurrence of low back pain is common: a prospective inception cohort study. J Physiother. 2019;65:159–165. doi: 10.1016/j.jphys.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Fayad F, Lefevre-Colau MM, Poiraudeau S, et al. Chronicité, récidive et reprise du travail dans la lombalgie: facteurs communs de pronostic [Chronicity, recurrence, and return to work in low back pain: common prognostic factors] Ann Readapt Med Phys. 2004;47:179–189. doi: 10.1016/j.annrmp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303:1295–1302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- 25.Martins R, Kotsopoulos N, Kosaner Kließ M, et al. Comparing the fiscal consequences of controlled and uncontrolled osteoarthritis pain applying a UK public economic perspective. J Health Econ Outcomes Res. 2021;8(1):127–136. doi: 10.36469/001c.24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieminen LK, Pyysalo LM, Kankaanpää MJ. Prognostic factors for pain chronicity in low back pain: a systematic review. Pain Rep. 2021;6(1):e919. doi: 10.1097/PR9.0000000000000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen TE, Karstof KI, Lauridsen HH, Manniche C. Trajectories of disability in low back pain. Pain Reports. 2022;7:e985. doi: 10.1097/PR9.0000000000000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham R, Mancher M, MillerWolman D. Clinical practice guidelines we can trust. Washington DC: Institute of Medicine. National Academies Press; 2011. [PubMed] [Google Scholar]
- 29.National Institute of health and care Excellence. 2014. The guidelines manual. Available at: https://www.nice.org.uk/process/pmg20/chapter/introduction. Accessed February 8, 2022.
- 30.Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Developing guidelines. BMJ. 1999;318:593e6. doi: 10.1136/bmj.318.7183.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzer W, LeBlanc FE, Dupuis M, et al. Scientific approach to the assessment and management of activity-related spinal disorders. Spine. 1987;12:1–58. [PubMed] [Google Scholar]
- 32.O’Connell NE, Chook CE, Wand BM, Ward SP. Clinical guidelines for low back pain: A critical review of consensus and inconsistencies across three major guidelines. Best Pract Res Clin Rheumatol. 2016;30:968–980. doi: 10.1016/j.berh.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Di Iorio D, Henley E, Doughty A. A survey of primary care physician practice patterns and adherence to acute low back problem guidelines. Arch Fam Med. 2000;9:1015–1021. doi: 10.1001/archfami.9.10.1015. [DOI] [PubMed] [Google Scholar]
- 34.Schers H, Braspenning J, Drijver R, Wensing M, Grol R. Low back pain in general practice: Reported management and reasons for not adhering to the guidelines in The Netherlands. Br J Gen Pract. 2000;50:640–644. [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanova J, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: The long road to guideline-concordant care. Spine J. 2011;11:622–632. doi: 10.1016/j.spinee.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Ramanathan SA, Hibbert PD, Maher CG, et al. CareTrack: toward appropriate care for low back pain. Spine (Phila Pa 1976). 2017;42:E802–9. doi: 10.1097/BRS.0000000000001972. [DOI] [PubMed] [Google Scholar]
- 37.Amonkar SJ, Dunbar AM. Do patients and general practitioners have different perceptions about the management of simple mechanical back pain? Int Musculoskelet Med. 2011;33:3–7. doi: 10.1179/175361511X12972993698186. [DOI] [Google Scholar]
- 38.Varrassi G, Moretti B, Pace MC, Evangelista P, Iolascon G. Common clinical practice from low back pain treatment: a modified Delphi study. Pain Ther. 2021;10:589–604. doi: 10.1007/s40122-021-00249-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163:e328–e332. doi: 10.1097/j.pain.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 40.Alonso-Coello P, Irfan A, Sola I, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Safety Health Care. 2010;19:e58. doi: 10.1136/qshc.2010.042077. [DOI] [PubMed] [Google Scholar]
- 41.Meroni R, Piscitelli D, Ravasio C, et al. Evidence for managing chronic low back pain in primary care: a review of recommendations from high-quality clinical practice guidelines. Disabil Rehabil. 2019;1:1–15. doi: 10.1080/09638288.2019.1645888. [DOI] [PubMed] [Google Scholar]
- 42.National Guideline Centre (UK) ‘Low back pain and sciatica in over 16s: assessment and management’. Manchester: National Institute for Health and Care Excellence; 2016. Available at: https://www.nice.org.uk/guidance/ng59.Last access on August 2nd 2022. [PubMed]
- 43.Toward Optimized Practice (TOP) Low Back Pain Working Group. Evidence-informed primary care management of low back pain: clinical practice guideline. Edmonton, AB: Toward Optimized Practice; 2015. Available from: https://actt.albertadoctors.org/CPGs/Lists/CPGDocumentList/LBP-guideline.pdf. Last access on August 2nd 2022.
- 44.Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine. 2009;34:1078–1093. doi: 10.1097/BRS.0b013e3181a103b1. [DOI] [PubMed] [Google Scholar]
- 45.Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34:1066–1077. doi: 10.1097/BRS.0b013e3181a1390d. [DOI] [PubMed] [Google Scholar]
- 46.Castellini G, Iannicelli V, Briguglio M, et al. Are clinical practice guidelines for low back pain interventions of high quality and updated? A systematic review using the AGREE II instrument. BMC Health Serv Res. 2020;20:970. doi: 10.1186/s12913-020-05827-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng JY, Mohiuddin U, Azizudin AM. Clinical practice guidelines for the treatment and management of low back pain: A systematic review of quantity and quality. Musculoskelet Sci Pract. 2021;51:102295. doi: 10.1016/j.msksp.2020.102295. [DOI] [PubMed] [Google Scholar]
- 48.Hanna M, Montero Matamala A, Perrot S, et al. Delivery of multimodal analgesia to effectively treat acute pain: a review from roma pain days. Cureus. 14:e22465. [DOI] [PMC free article] [PubMed]
- 49.Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27:2791–2803. doi: 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- 50.Qaseem A, Wilt TJ, McLean RM, Forciea MA, for the clinical guidelines committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Annals Intern Med. 2017;166:514–530. [DOI] [PubMed]
- 51.Zis P, Daskalaki A, Bountouni I, Sykioti P, Varrassi G, Paladini A. Depression and chronic pain in the elderly: Links and management challenges. Clin Inter Aging. 2017;12:709–720. doi: 10.2147/CIA.S113576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verhagen AP, Downie A, Popal N, Maher C, Koes BW. Red flags presented in current low back pain guidelines: a review. Eur Spine J. 2016;25:2788–2802. doi: 10.1007/s00586-016-4684-0. [DOI] [PubMed] [Google Scholar]
- 53.Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384:1586–1596. doi: 10.1016/S0140-6736(14)60805-9. [DOI] [PubMed] [Google Scholar]
- 54.Panella L. Rinonapoli G, Coaccioli S. Where should analgesia lead to? Quality of life and functional recovery with tapentadol. J Pain Res. 2019;12:1561–1567. doi: 10.2147/JPR.S190158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Boekel RLM, Vissers KCP, van der Sande R, et al. Moving beyond pain scores: Multidimensional pain assessment is essential for adequate pain management after surgery. PLoS ONE. 2017;12:e0177345. doi: 10.1371/journal.pone.0177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiarotto A, Deyo RA, Terwee CB, et al. Core outcome domains for clinical trials in non-specific low back pain. Eur Spine J. 2015;24:1127–1142. doi: 10.1007/s00586-015-3892-3. [DOI] [PubMed] [Google Scholar]
- 57.O'Sullivan K, O'Keeffe M, O'Sullivan P. NICE low back pain guidelines: opportunities and obstacles to change practice. Br J Sports Med. 2017;51:1632–1633. doi: 10.1136/bjsports-2017-097810. [DOI] [PubMed] [Google Scholar]
- 58.Sudlow A, le Roux CW, Pournaras DJ. Review of multimodal treatment for type 2 diabetes: combining metabolic surgery and pharmacotherapy. Ther Adv Endocrinol Metab. 2019;10:2042018819875407. doi: 10.1177/2042018819875407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sudlow A, W le Roux C, J Pournaras D. Review of Advances in Anti-obesity Pharmacotherapy: Implications for a Multimodal Treatment Approach with Metabolic Surgery. Obes Surg. 2019;29:4095–4104. [DOI] [PubMed]
- 60.Romeyke T, Noehammer E, Stummer H. Patient-reported outcomes following inpatient multimodal treatment approach in chronic pain-related rheumatic diseases. Global Adv Health Med. 2020;9:1–12. doi: 10.1177/2164956120948811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bochner B, Scardino P. Multimodal therapy for urologic cancers. Nat Rev Urol. 2006;3:453. doi: 10.1038/ncpuro0583. [DOI] [PubMed] [Google Scholar]
- 62.Chaer RA, Dayal R, Lin SC, et al. Multimodal therapy for acute and chronic venous thrombotic and occlusive disease. Vasc Endovascular Surg. 2005;39(5):375–80. doi: 10.1177/153857440503900501. [DOI] [PubMed] [Google Scholar]
- 63.Kenneth L. Kirsh, Scott M. Fishman, MD, Multimodal approaches to optimize outcomes of chronic opioid therapy in the management of chronic pain. Pain Med 2011;12 (suppl_1):S1–S11. [DOI] [PubMed]
- 64.Mescouto K, Olson RE, Hodges PW, Setchell J. A critical review of the biopsychosocial model of low back pain care: time for a new approach? Disabil Rehabil. 2022;44(13):3270–3284. doi: 10.1080/09638288.2020.1851783. [DOI] [PubMed] [Google Scholar]
- 65.Lang E, Kastner S, Liebig K, Neundorfer B. Interventions for improvement of primary care in patients with low back pain: how effective are advice to primary care physicians on therapies and a multimodal therapy program arising out of cooperation of outpatient health care structures? Schmerz. 2002;16:22–33. doi: 10.1007/s004820100091. [DOI] [PubMed] [Google Scholar]
- 66.Kohles S, Barnes D, Gatchel RJ, Mayer TG. Improved physical performance outcomes after functional restoration treatment in patients with chronic low-back pain. Early versus recent training results. Spine. 1990;15:1321–1324. doi: 10.1097/00007632-199012000-00016. [DOI] [PubMed] [Google Scholar]
- 67.Luk KD, Wan TW, Wong YW, et al. A multidisciplinary rehabilitation programme for patients with chronic low back pain: a prospective study. J Orthop Surg (Hong Kong) 2010;18:131–138. doi: 10.1177/230949901001800201. [DOI] [PubMed] [Google Scholar]
- 68.Borys C, Lutz J, Strauss B, Altmann U. Effectiveness of a multimodal therapy for patients with chronic low back pain regarding pre-admission healthcare utilization. PLoS ONE. 2015;10(11):e0143139. doi: 10.1371/journal.pone.0143139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Migliorini F, Maffulli N, Eschweiler J, et al. The pharmacological management of chronic lower back pain. Expert Opin Pharmacother. 2021;22(1):109–119. doi: 10.1080/14656566.2020.1817384. [DOI] [PubMed] [Google Scholar]
- 70.Giordan E, Billeci D, Del Verme J, Varrassi G, Coluzzi F. Endoscopic transforaminal lumbar foraminotomy: a systematic review and meta-analysis. Pain Ther. 2021;10(2):1481–1495. doi: 10.1007/s40122-021-00309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Briggs AM, Slater H, Hsieh E, et al. System strengthening to support value-based care and healthy ageing for people with chronic pain. Pain. 2019;160:1240–1244. doi: 10.1097/j.pain.0000000000001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romeyke T, Noehammer E, Stummer H. Ensuring quality in interdisciplinary inpatient chronic care. SAGE Open. 2020;10:2158244020914654. doi: 10.1177/2158244020914654. [DOI] [Google Scholar]
- 73.Varrassi G, Alon E, Bagnasco M, et al. Towards an effective and safe treatment of inflammatory pain: a Delphi-guided expert consensus. Adv Ther. 2019;36:2618–2637. doi: 10.1007/s12325-019-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roelofs PD, Deyo RA, Koes BW, et al. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev. 2008;23:CD000396. [DOI] [PMC free article] [PubMed]
- 75.Shaheed CA, Ferreira G, Dmitritchenko A, et al. The efficacy and safety of paracetamol for pain relief: an overview of systematic reviews. MJA. 2021;214:324–331. doi: 10.5694/mja2.50992. [DOI] [PubMed] [Google Scholar]
- 76.Saragiotto BT, Machado GC, Ferreira ML, et al. Paracetamol for low back pain. Cochrane Database System Rev. 2016;6:CD012230. [DOI] [PMC free article] [PubMed]
- 77.Anderson DB, Shaheed CA. Medications for treating low back pain in adults. Evidence for the use of paracetamol, opioids, nonsteroidal anti-inflammatories, muscle relaxants, antibiotics, and antidepressants: an overview for musculoskeletal clinicians. J Orthop Sports Phys Ther. 2022;52(7):425–431. [DOI] [PubMed]
- 78.Papa A, Di Dato MT, Lo Bianco G, Gazzerro G, Salzano AM, Di Costanzo E, Tammaro D, Schatman ME, Varrassi G. Intraarticular STP radiofrequency for painful osteoarthritis in the knee: a retrospective single center analysis. J Pain Res. 2021;14:2441–2447. doi: 10.2147/JPR.S317569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veizi E, Hayek S. Interventional therapies for chronic low back pain. Neuromodulation. 2014;17:31–45. doi: 10.1111/ner.12250. [DOI] [PubMed] [Google Scholar]
- 80.Johnson SM, Hutchins T, Peckham M, et al. Effects of implementing evidence-based appropriateness guidelines for epidural steroid injection in chronic low back pain: the EAGER (Esi Appropriateness GuidElines pRotocol) study. BMJ Open Quality. 2019;8:e000772. doi: 10.1136/bmjoq-2019-000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayek SM, McEwan MT, Veizi E, DeLozier SJ, Pogrebetskaya M. Effects of bupivacaine on opioid patient-controlled intrathecal analgesia in chronic pain patients implanted with drug delivery systems. Pain Med. 2021;22(1):22–33. doi: 10.1093/pm/pnaa076. [DOI] [PubMed] [Google Scholar]
- 82.Patel VB, Wasserman R, Imani F. Interventional therapies for chronic low back pain: a focused review (efficacy and outcomes) Anesth Pain Med. 2015;5:e29716. doi: 10.5812/aapm.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Varrassi G, Hanna M, Macheras G, Montero A, Montes Perez A, Meissner W, Perrot S, Scarpignato C. Multimodal analgesia in moderate-to-severe pain: a role for a new fixed combination of dexketoprofen and tramadol. Curr Med Res Opin. 2017;33(6):1165–1173. doi: 10.1080/03007995.2017.1310092. [DOI] [PubMed] [Google Scholar]
- 84.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22:588–593. doi: 10.1097/ACO.0b013e328330373a. [DOI] [PubMed] [Google Scholar]
- 85.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 86.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthr Rheumatol. 2020;72:220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Varrassi G, Yeam CT, Rekatsina M, et al. The Expanding role of the COX inhibitor/opioid receptor agonist combination in the management of pain. Drugs. 2020;80:1443–1453. doi: 10.1007/s40265-020-01369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wright M and Lee JA. Multimodal analgesia and discharge opioid requirements in burn patients. J Burn Care Res 2020;41:963–966; iraa088. [DOI] [PMC free article] [PubMed]
- 89.Hamrick KL, Beyer CA, Lee JA, et al. Multimodal analgesia, and opioid use in critically ill trauma patients. J Am Coll Surg. 2019;228:769–775. doi: 10.1016/j.jamcollsurg.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 90.Walkerly A, Neugebauer RE, Misko B, et al. Prevalence, predictors, and trends of opioid prescribing for lower back pain in United States emergency departments. J Clin Pharm Ther. 2021;46:698–704. doi: 10.1111/jcpt.13324. [DOI] [PubMed] [Google Scholar]
- 91.Anderson SW, Bhattacharjee S, Patanwala AE. Effect of opioid analgesics on emergency department length of stay among low back pain patients in the United States. Am J Emerg Med. 2020;38:1802–1806. doi: 10.1016/j.ajem.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 92.Barakat A. Revisiting tramadol: a multi-modal agent for pain management. CNS Drugs. 2019;33:481–501. doi: 10.1007/s40263-019-00623-5. [DOI] [PubMed] [Google Scholar]
- 93.Martinez L, Ekman E, Nakhla N. Perioperative opioid-sparing strategies: utility of conventional NSAIDs in adults. Clin Ther. 2019;41:2612–2628. doi: 10.1016/j.clinthera.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 94.Langford R, Morte A, Sust M, Cebrecos J, Vaqué A, Ortiz E, Fettiplace J, Adeyemi S, Raba G, But-Husaim L, Gascón N, Plata-Salamán C. Efficacy and safety of co-crystal of tramadol-celecoxib (CTC) in acute moderate-to-severe pain after abdominal hysterectomy: A randomized, double-blind, phase 3 trial (STARDOM2) Eur J Pain. 2022;26(10):2083–2096. doi: 10.1002/ejp.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scott LJ, Perry CM. Tramadol: a review of its use in perioperative pain. Drugs. 2000;60:139–176. doi: 10.2165/00003495-200060010-00008. [DOI] [PubMed] [Google Scholar]
- 96.Vazzana M, Andreani T, Fangueiro J, et al. Tramadol hydrochloride: Pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed Pharmacother. 2015;70:234–238. doi: 10.1016/j.biopha.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 97.Lasko B, Levitt RJ, Rainsford KD, Bouchard S, Rozova A, Robertson S. Extended-release tramadol/paracetamol in moderate-to-severe pain: a randomized, placebo-controlled study in patients with acute low back pain. Curr Med Res Opin. 2012;28(5):847–857. doi: 10.1185/03007995.2012.681035. [DOI] [PubMed] [Google Scholar]
- 98.Klosterhalfen S, Enck P. Psychobiology of the placebo response. Auton Neurosci. 2006;125:94–99. doi: 10.1016/j.autneu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 99.Miller FG, Rosenstein DL. The nature and power of the placebo effect. J Clin Epidemiol. 2006;59:331–335. doi: 10.1016/j.jclinepi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 100.Moore RA, Gay-Escoda C, Figueiredo R, et al. Dexketoprofen/tramadol: randomised double-blind trial and confirmation of empirical theory of combination analgesics in acute pain. J Headache Pain. 2015;16:541. doi: 10.1186/s10194-015-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moore RA, McQuay HJ, Tomaszewski J, et al. Dexketoprofen/tramadol 25 mg/75 mg: randomised double-blind trial in moderate-to-severe acute pain after abdominal hysterectomy. BMC Anesthesiol. 2016;16:9. doi: 10.1186/s12871-016-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gay-Escoda C, Hanna M, Montero A, et al. Tramadol/dexketoprofen (TRAM/DKP) compared with tramadol/paracetamol in moderate to severe acute pain: results of a randomised, double-blind, placebo and active-controlled, parallel group trial in the impacted third molar extraction pain model (DAVID study) BMJ Open. 2019;9:e023715. doi: 10.1136/bmjopen-2018-023715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varrassi G, Coaccioli S, De-Andrés J, et al. Expert consensus on clinical use of an orally administered dexketoprofen plus tramadol fixed-dose combination in moderate-to-severe acute pain: a Delphi study. Adv Ther. 2019;36:3174–3185. doi: 10.1007/s12325-019-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meloncelli S, Divizia M, Germani G. Efficacy and tolerability of orally administered tramadol/dexketoprofen fixed-dose combination compared to diclofenac/thiocolchicoside in acute low back pain: experience from an Italian, single-centre, observational study. Curr Med Res Opin. 2020;36:1687–1693. doi: 10.1080/03007995.2020.1814228. [DOI] [PubMed] [Google Scholar]
- 105.Perna A, Ricciardi L, Barone G, et al. Medical management of acute non-specific low back pain: comparison of different medical treatments, one center’s retrospective analysis. J Biol Reg Homeo Agents. 2018;32:121–129. [PubMed] [Google Scholar]
- 106.Varrassi G, Hanna M, Coaccioli S, et al. DANTE Study: The first randomized, double-blind, placebo and active-controlled, parallel arm group study evaluating the analgesic efficacy and safety of Dexketoprofen trometamol and Tramadol hydrochloride oral fixed dose combination on moderate to severe acute pain in patients with acute low back pain: Rationale and design. Pain Ther. 2022;11(3):1055–1070. doi: 10.1007/s40122-022-00407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.https://bit.ly/3zaXKOk WHO July 2022 (accessed August 20, 2022)
- 108.Corp N, Mansell G, Stynes S, et al. Evidence-based treatment recommendations for neck and low back pain across Europe: A systematic review of guidelines. Eur J Pain. 2021;25:275–295. doi: 10.1002/ejp.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pantaleon L. Why measuring outcomes is important in health care. J Vet Intern Med. 2019;33:356–362. doi: 10.1111/jvim.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fourré A, Fierens A, Michielsen J, Ris L, Dierick F, Roussel N. An interactive e-learning module to promote bio-psycho-social management of low back pain in healthcare professionals: a pilot study. J Man Manip Ther. 2022;30(2):105–115. doi: 10.1080/10669817.2021.1988397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.