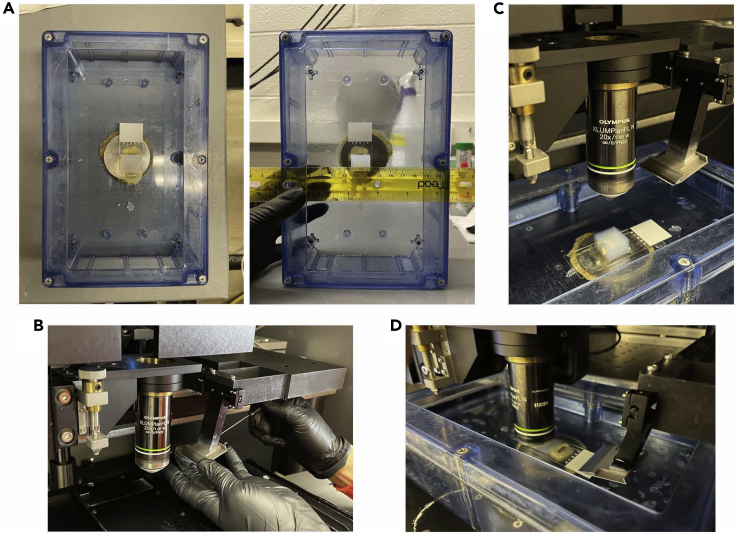
Figure 8.
Setting up the STPT sample chamber and automated vibratome
(A) STPT sample buffer chamber with an embedded brain sample already glued to a magnetic glass slide for imaging. It is helpful to use a ruler to align the posterior end of the brain so that it is straight.
(B) Replacing the vibratome blade on the holder requires careful handling as the blade is sharp. Two small hex screws are used to securely fasten the holder around the blade.
(C) After a fresh blade is put in the holder, the sample buffer chamber is filled with 0.05 M phosphate buffer and placed in the TissueCyte directly underneath the 20× objective lens and the vibratome.
(D) During sectioning, the objective lens should be submerged in the buffer with no trace of bubbles and the blade should start vibrating a few centimeters away from the edge of the oxidized agarose sample block. It is important that the sectioning quality is consistent at 50 μm before image acquisition.