Structured Abstract
Purpose:
Although ratings of perceived exertion (RPE) are widely used to guide exercise intensity in cardiac rehabilitation (CR), it is unclear if target heart rate ranges (THRR) can be implemented in CR programs that predominantly using RPE and what impact this has on changes in exercise capacity.
Methods:
We conducted a three-group pilot randomized control trial (#NCT03925493) comparing RPE of 3–4 on the 10-point modified Borg Scale, 60–80% of heart rate reserve (HRR) with heart rate (HR) monitored by telemetry, or 60–80% of HRR with a personal heart rate monitor (HRM) for high fidelity adherence to THRR. Primary outcomes were protocol fidelity and feasibility. Secondary outcomes included exercise HR, RPE, and changes in functional exercise capacity.
Results:
Of 48 participants randomized, 4 patients dropped out, 20 stopped prematurely (COVID-19 pandemic), and 24 completed the protocol. Adherence to THRR was high regardless of HRM, and patients attended a median (IQR) of 33 (23,36) sessions with no difference between groups. After randomization, HR increased by 1±6, 6±5, and 10±9 bpm (p=.02); RPE (average score 3.0 ±.05) was unchanged, and functional exercise capacity increased by 1.0±1.0, 1.9±1.5, 2.0 ±1.3 workload METs (effect size between groups, ηp2 =.11, p=.20) for the RPE, THRR, and THRR+HRM groups, respectively.
Conclusions:
We successfully implemented THRR in an all-RPE CR program without needing a HRM. Patients randomized to THRR had higher exercise HRs but similar RPE ratings. THRR may be preferable to RPE in CR populations for fitness gains, but this needs confirmation in an adequately powered trial.
Keywords: Target heart rate, rate of perceived exertion, cardiac rehabilitation, stress test, METS
Condensed Abstract
Both THRR and RPE based exercise prescriptions are widely used in CR, it is unclear if one method leads to better outcomes in CR. We found that patients randomized to THRR had higher exercise HRs but similar RPE ratings. THRR appears preferable to RPE in CR populations for fitness gains.
INTRODUCTION
Exercise is a core component of cardiac rehabilitation (CR). Exercise training alone reduces total and cardiovascular mortality by 27% and 31%, respectively. Most of the benefits of CR are attributable to improvements in cardiorespiratory fitness (CRF).1–5 Exercise intensity appears to be the most important factor in improving cardiorespiratory fitness, which is inversely related with all-cause mortality among patients with heart disease.6 Recent studies have suggested that common methods to guide exercise intensity in CR may produce little to no change in exercise capacity.7–9 If true, current methods of prescribing exercise intensity in CR may need to be reexamined.
Many methods to guide exercise intensity are used in CR, but exactly which method leads to the greatest improvements in CRF while minimizing adverse effects is unknown.3 There are two predominate methods of developing an exercise prescription in CR; exercise based on perceived effort and exercise based on heart rate (HR). The American Association of Cardiovascular and Pulmonary Rehab (AACVPR) 6th edition guidelines recommend both ratings of perceived exertion (RPE) and target heart rate range (THRR) based exercise prescription as standards of care in CR, but rigorous comparative studies are few. Recent retrospective studies have suggested that performing maximal exercise testing early in CR may allow better tailoring of an exercise prescription and thus increase exercise gains.10,11
We conducted a randomized controlled pilot trial to compare exercise prescriptions based on RPE or THRR to assess if we could 1) recruit an adequate number of patients to support a larger trial, 2) implement a THRR exercise prescription, and lastly, 3) estimate effect sizes for the change in exercise capacity.
METHODS
Trial Design
This pilot study was a single-site, prospective, open-label, randomized controlled trial (clinical.trials.gov#NCT03925493), that included three parallel arms, comparing RPE, THRR, and THRR with a personal heart rate monitor (HRM, THRR+HRM). Patients were randomized 1:1:1 to the RPE, THRR, or THRR+HRM. We included a THRR+HRM group to assure a high-fidelity intervention to the prescribed THRR in a program that almost exclusively used RPE. This study was approved by the institutional review boards of both Baystate Medical Center and Springfield College.
Participants
Patients referred to CR with a diagnosis of percutaneous coronary intervention (PCI), myocardial infarction (MI), or coronary artery bypass graft (CABG) were eligible. Subjects were excluded for any condition that would limit exercise intensity or making monitoring an exercise HR technically difficult (SDC, Supplemental Digital Content). We further excluded patients at high risk of non-adherence or early-drop out, including patients with plans to undergo elective surgery or a clinically indicated stress test after enrolling in CR, or patients that planned to attend fewer than 12 CR sessions.12 We aimed to enroll 20 patients per group to test our protocols and measure preliminary effect sizes.13,14
Baseline Assessments and Randomization
Patients interested in participating signed informed consent, typically during the 1st or 2nd session of CR. Comorbidities were recorded from the medical record along with medications and metoprolol equivalent doses of beta blockers.15 Patients were given a token incentive for participation (SDC).
RedCap, a secure web-based database platform, was used to randomize patients in a sequential and random fashion, as well as store and export data. Groups were blocked by the presence of thoracic surgery, because CABG and valve patients can have post-surgery anemia, tend to be more deconditioned due to longer hospitals stays, have greater gains in exercise capacity, and have different exercise restrictions compared to MI and PCI patients.7 Due to the nature of the intervention, all assignments were open label, including patients and CR staff.
During baseline sessions 1–3 of CR, resting HR, and exercise RPE were measured in both groups. Metabolic equivalence of tasks (workload METs), as measured by exercise training workload on a treadmill, was measured during the third session of CR.16 RPE was used to assess baseline exercise effort.
Exercise Prescription and Progression
All subjects, regardless of group assignment did approximately 30–40 min of aerobic exercise typically on the treadmill or upright bicycle. Only exercise intensity differed between groups. During each CR session, staff reassessed each patient’s exercise workload and, if appropriate, increased exercise workload in accordance with the exercise prescription method (SDC).
Control group
Patients randomized to the RPE group were asked to exercise between a RPE of 3–4 on the 10-point modified Borg scale for all 36 sessions of CR. We followed best practices for using RPE, which included posting the RPE scale in the gym, asking patients to provide a RPE rating during exercise based on whole-body perceived effort, and we provided physiological feedback when appropriate.17,18
Intervention Groups
Within 5–10 days of randomization, patients assigned to THRR or THRR+HRM underwent a baseline symptom limited maximal exercise test, typically before the 5th session of CR. Patients took all medications on the day of the exercise test. Using the subjects peak and resting HRs measured during the maximal exercise test, a THRR was calculated based on 60–80% of heart rate reserve (HRR), which was used to guide exercise progression for the rest of CR, beginning on the next session. Patients typically started exercising at the lower end of this range (about 60% HRR) and progressed to the upper end of this range (80% HRR) by the end of CR. RPE was measured in this group for every session, but it was not used to guide exercise intensity.
Subjects in the RPE group did not undergo a maximal exercise test because we were concerned an exercise test might improve the subject’s confidence in their ability to exercise and subsequently lead to exercising at a higher intensity. Moreover, the use of RPE without a maximal exercise test is the most common pattern of exercise prescription in CR programs in the US.19,20 The RPE group was trained based on RPE not on THRR. Thus, in the RPE group, HR was measured solely as an outcome and RPE was used as the training parameter. Alternately, in the THRR groups, HR was used as the training parameter, and RPE was measured as an outcome. Finally, because all patients were randomized, we assumed that baseline exercise capacity would be similar between groups.
Subjects randomized to the THRR+HRM group were given a Polar A370 and H10 HRM. Patients were instructed on how to use the HRM and were aware that the HRM was to be used to provide rapid HR feedback during CR. Patients were instructed to wear the HRM during every CR session. No instructions were provided to patients on whether to wear or use the HRM device at home.
Measurements and Outcomes
The primary goals of this study were to enroll 60 patients, prescribe exercise using a THRR, assure adherence to THRR, and retain patients in CR for at least 12 CR sessions. Secondary outcomes included peak exercise capacity (VO2peak) at the end of CR, change in exercise HR, RPE, adjustments in exercise workload (duration or intensity of exercise), and change in workload METS at program completion. Process outcomes included retaining 90% of subjects for ≥12 CR sessions, subject’s adherence to their THRR for ≥7 minutes for 90% of CR sessions, and lastly have 90% of subjects complete the exercise test within the first 4 sessions of CR (SDC).
For each CR session, we noted exercise HR, change in HR from rest, reported RPE, and frequency of upward adjustments in exercise workload. To examine adherence to the THRR, we noted daily percent of HRR based on subjects exercising HR per CR session. When measuring HR, we used either the HRM (THRR+HRM) or gym equipment (RPE and THRR) to measure HR when telemetry was not available (SDC).
At the end of CR, all subjects regardless of group assignment, completed a cardiopulmonary exercise test (CPX) on a treadmill using either a Bruce or modified Bruce protocol to measure CRF. The CPX was completed using the VMAX Cart Vyntus within the last 6 CR sessions and the stress lab staff were blinded to group assignment. Consistent with AACVPR performance measures, the change in workload METs at exercise training workload, was calculated for all patients from the 3rd to the last CR session.21,22
All patients were given a satisfaction survey at the end of CR, regardless of whether they completed all aspects of the protocol. Two authors (MS, QP) developed the survey questions, which consisted of multiple choice, Likert scale, and open-ended questions (SDC).
In response to the COVID-19 pandemic, the CR program and exercise testing laboratory closed on March 16th, 2020. Due to these closures, not all enrolled patients were able to complete the protocol.
Statistical Analysis
Data for all patients were averaged and inspected for patterns between groups and overtime, regardless of missing data and COVID-related drop out (Table 1). All data was analyzed using descriptive statistics, regardless of program adherence. However, because of the missing data due to COVID, we limited formal statistical analysis and testing to patients who completed ≥11 sessions of CR after randomization. This cut-off allowed us to include most of the subjects that underwent the intervention, while allowing a reasonable amount of time for the intervention to take effect. All analyses were otherwise done using intent-to-treat principles, including patients who dropped out of CR for non-COVID-19 related reasons (SDC).
Table 1.
Baseline Patient Characteristics by Group
| RPE N = 16 | THRR N= 16 | THRR+HRM N = 16 | P | |
|---|---|---|---|---|
|
| ||||
| Males | 11 (69) | 12 (75) | 12 (75) | 0.91 |
| Age (yrs) | 67 ± 6 | 69 ± 8 | 65 ± 7 | 0.19 |
| Weight (kg) | 83 ± 21 | 81 ± 15 | 79 ± 19 | 0.63 |
| BMI | 30 ± 5 | 28 ± 5 | 30 ± 5 | 0.63 |
| Diagnosis | ||||
| PCI | 10 (63) | 10 (63) | 10 (21) | 1.0 |
| CABG | 6 (38) | 6 (38) | 6 (13) | |
| Comorbidities | ||||
| Smoker | 2 (13) | 2 (13) | 1 (0) | 0.81 |
| HTN | 14 (88) | 13 (81) | 14 (88) | 0.85 |
| Diabetes | 7 (44) | 6 (38) | 4 (25) | 0.55 |
| Kidney Disease | 0 (0) | 2 (13) | 1 (6) | 0.36 |
| Hyperlipidemia | 15 (94) | 13 (81) | 14 (88) | 0.58 |
| PAD | 1 (6) | 1 (6) | 1 (6) | 1.0 |
| Lung Disease | 0 (0) | 1 (6) | 2 (13) | 0.36 |
| Medications | ||||
| Beta Blocker | 15 (94) | 15 (94) | 15 (94) | 1.0 |
| Metoprolol equivalent dose (mg) | 43 ± 26 | 41 ± 32 | 48 ± 83 | 0.76 |
| Aspirin | 15 (94) | 16 (100) | 16 (100) | 0.38 |
| Statin | 14 (88) | 15 (94) | 14 (88) | 0.36 |
| Anti-platelet | 11 (69) | 15 (94) | 11 (69) | 0.16 |
| Baseline RHR (BPM) | 77 ± 8 | 70 ± 6 | 74 ± 12 | 0.49 |
| Baseline METS | 3.1 ± 0.6 | 3.4 ± 1.4 | 3.4 ± 0.9 | 0.26 |
| Number of CR sessions Prior to GXT | 4 ± 3 | 4 ± 1 | 0.19 | |
| Change in METS | 1.5 ± 1 | 1.8 ± 1.3 | 2.4 ± 1.0 | 0.20 |
Units: Age, yrs; kg, kilograms; mg, milligrams; Abbreviations: BMI: Body mass index; BPM, beats per minute; CABG, Coronary artery bypass graft; HRM, heart rate monitor; HTN, Hypertension; METs, Metabolic equivalence of task; N, number of subjects; PAD, peripheral artery disease; PCI, Percutaneous coronary intervention; RHR, resting heart rate; RPE, rate of perceived exertion; THRR, target heart rate range [Data are presented as n (%)]
To calculate an equivalent dose of metoprolol we used the following conversion: Coreg is 4 X more potent than metoprolol, multiply coreg by 4, Bisoprolol is 5 times more potent than metoprolol, multiply by 5, and atenolol is 2 times more potent than metoprolol, multiply by 2.
Using this “per-protocol” group (completing ≥11 sessions after randomization), we determined the frequency of adjustments to workload, change in exercise HR, change in HR from rest, RPE, and workload METS. To allow for a repeated measure analysis, we created 3-time intervals: baseline, time 1 (T1), and time 2 (T2) which incorporated the first 4 sessions of CR (baseline), and then two sequential periods of 5 CR session each (T1 and T2).
RESULTS
Participants
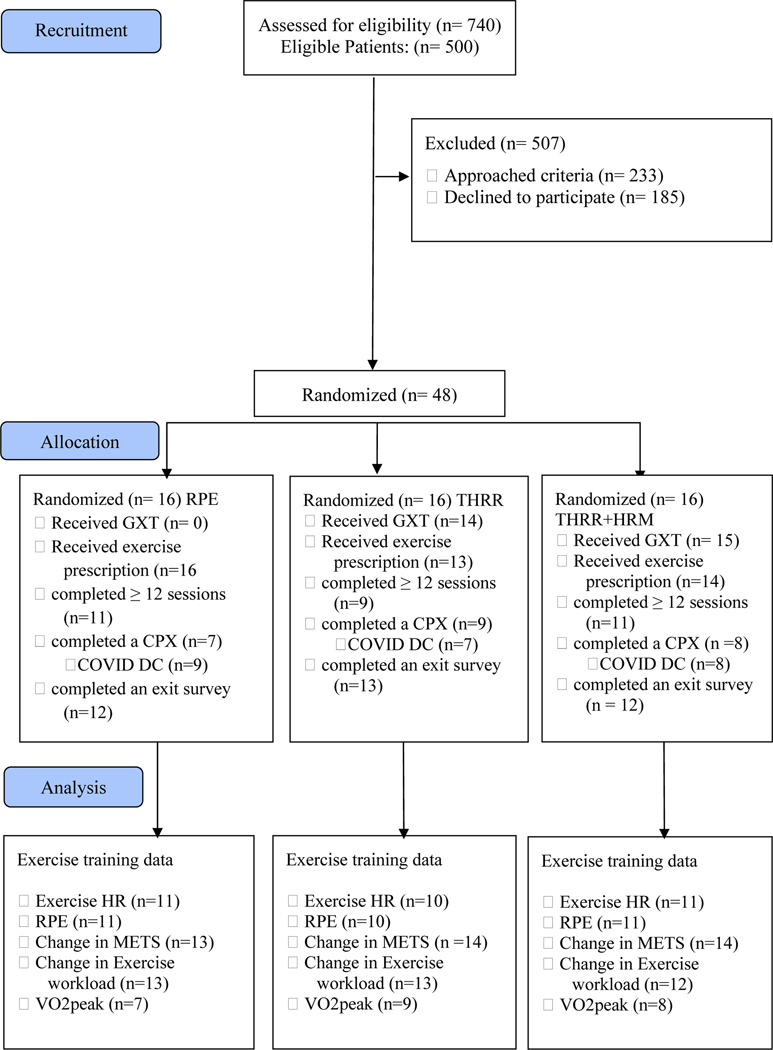
A total of 500 patients were eligible, 220 were approached, and a total of 48 (22% of approached) subjects were enrolled (Figure 1). Patients with unstable angina, high copays, chronic atrial fibrillation, and pacemakers were not approached. Due to the pandemic, we were unsuccessful in enrolling all planned 60 patients. Baseline characteristics are shown in Table 1. There were no differences between groups for age, initial weight, BMI, or beta blocker equivalent doses. Importantly, baseline workload METS (Table 1) were balanced between groups and was used as the baseline measure for changes in exercise workload.
Figure 1.
Consort diagram. Abbreviations: CPX, cardiopulmonary exercise test; DC, drop out; GXT, graded exercise test; HR, heart rate; HRM, heart rate monitor; METS, metabolic equivalence of tasks; n, number of subjects; RPE, rate of perceived exertion; THRR, target heart rate range; VO2peak, peak oxygen consumption
Adherence and Descriptive Outcomes
Of the 48 who began the study, 24 completed the protocol, 20 were discharged early due to the COVID-19 pandemic, and 4 patients dropped out (Figure 1). In total, 24 subjects completed all assessments, including the final CPX analysis (Table 2). The median number of sessions attended for all subjects was 26 (20,36). The median number of sessions for subjects that were not discharged early due to COVID-19 was 33 (23,36) with most subjects (89%) completing at least 12 CR sessions.
Table 2.
Key Cardiopulmonary Variables during the completion Cardiopulmonary Test
| RPE N = 7 | THRR N = 9 | THRR+HRM N = 8 | P | ηp2 | |
|---|---|---|---|---|---|
|
| |||||
| VO2peak (ml/kg/min) | 19 ± 4 | 19 ± 6 | 26 ± 8 | 0.13 | 0.13 |
| Peak METS | 9 ± 2 | 10 ± 3 | 12 ± 4 | 0.11 | 0.19 |
| Peak HR (bpm) | 122 ± 19 | 125 ± 14 | 136 ± 17 | 0.27 | 0.12 |
| RER | 1.1 ± 0.17 | 1.2 ± 0.11 | 1.2 ± 0.15 | 0.49 | 0.07 |
| Total Time (min) | 7.3 ± 2.3 | 8.3 ± 3.1 | 10.6 ± 2.2 | 0.053 | 0.02 |
Units: Peak HR, bpm; total time, minutes; VO2peak, ml/kg/min; Abbreviations: BPM, beats per minute; ηp2, eta squared; HR, Heart rate, bpm, HRM, heart rate monitor; METS, Metabolic equivalence of task; Min, Minute; N, number of subjects; p, probability value; RER, respiratory exchange ratio; RPE, rate of perceived exertion; THRR, target heart rate range; VO2peak, peak oxygen consumption
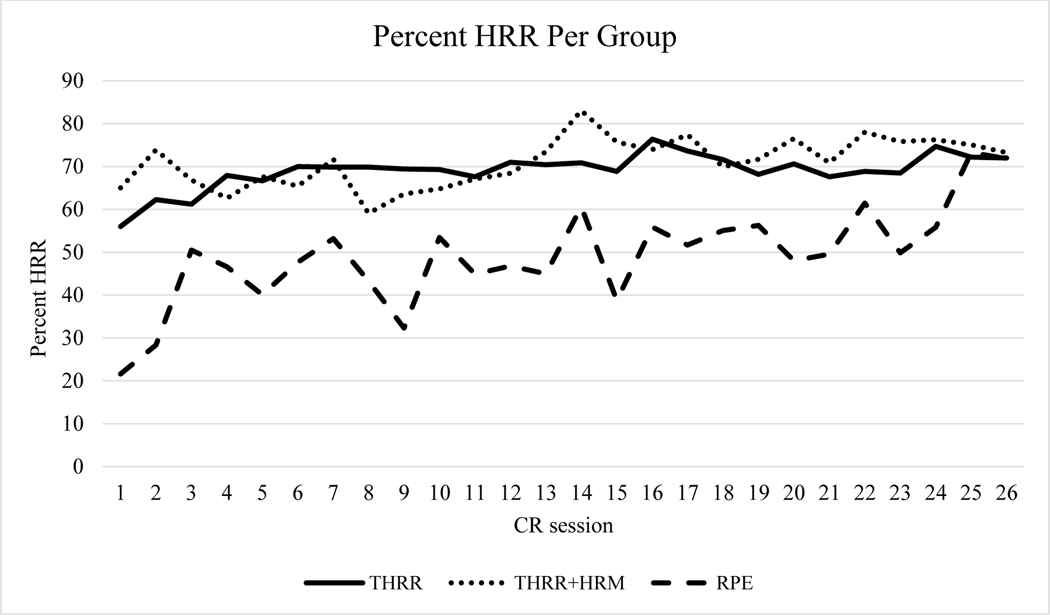
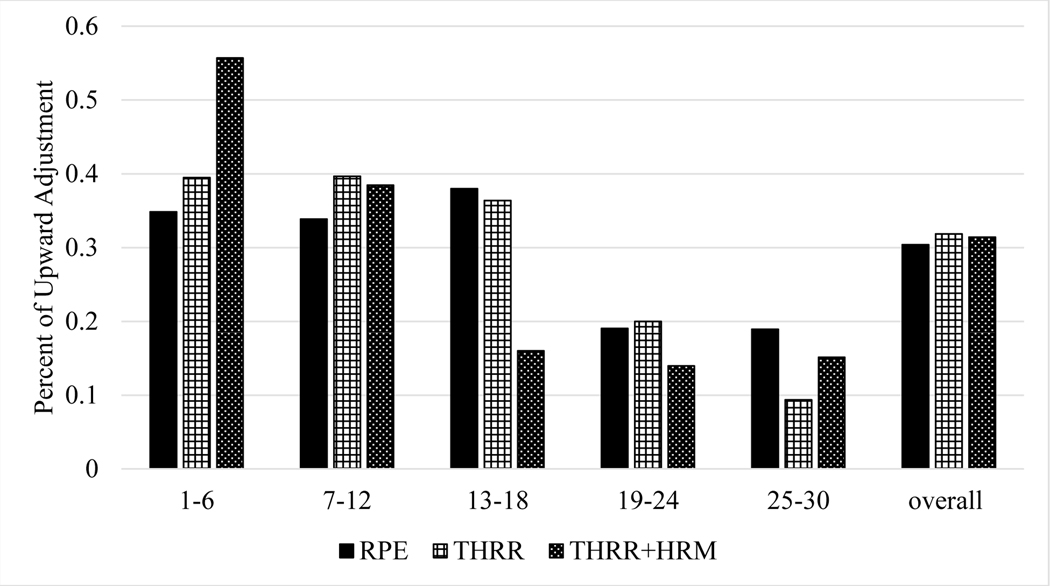
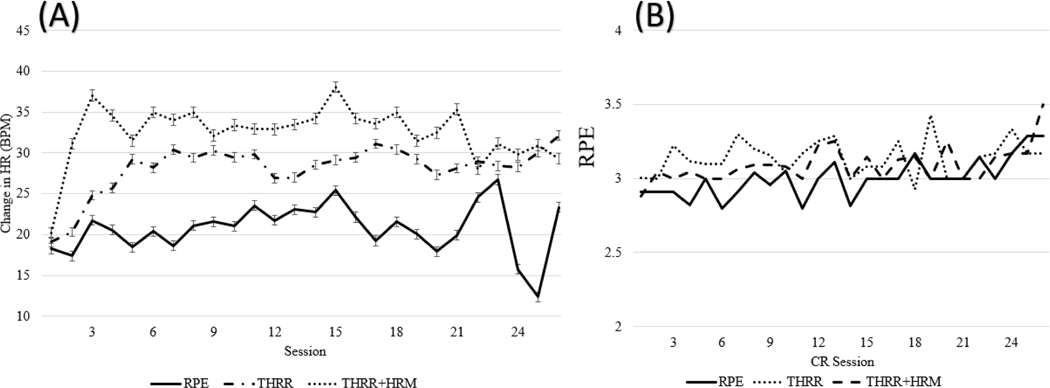
Using all available data, subjects exercised within their THRR for 83±11 and 89±12% of CR sessions for the THRR and THRR+HRM groups, respectively (Figure 2). We found that, within the first 6 sessions of CR, patients in the THRR and THRR+HRM group had more frequent increases in workload but then had fewer increases later in CR (Figure 3). Finally, we noted significantly higher exercising HRs between both THRR groups and RPE group (Figure 4A,B).
Figure 2.
Percent of HRR calculated from peak exercise heart rate for each CR session. Subjects in the THRR groups successfully exercised within 60–80% of HRR throughout CR. Subjects in the RPE group exercised closer to 50% of their calculated HRR. Subjects in the RPE groups HRR calculation can be found in the supplemental digital content. HRM: Heart rate monitor HRR; Heart rate reserve; RPE, rate of perceived exertion; THRR: target heart rate range.
Figure 3.
The percent of upward adjustments per 6 session intervals across groups. As noted, patients in the in THRR and THRR + HRM groups, had more frequent upward adjustments in workload earlier in CR compared to subjects in the RPE group. Data was averaged over 6 session intervals to allow for adjustments to be seen after care plans were completed every 6th session. Abbreviations: CR, cardiac rehab; HRM, heart rate monitor; RPE, rate of perceived exertion; THRR, target heart rate.
Figure 4A.
The average change in HR from rest across CR sessions per exercise method. As seen, there was a notable difference between groups across sessions. Figure 4B. Average RPE across CR sessions per each exercise prescription method. As seen, there were no clear difference between groups for RPE. Units: BPM, beats per minute; Abbreviations: CR, Cardiac rehab; HR, Heart rate; HRM, heart rate monitor; RPE, Rate of perceived exertion; THRR, target heart rate range
Formal Statistical Analysis
In addition to the 24 subjects who completed the full protocol, we included 8 subjects that were discharged early due to COVID-19 in formal statistical testing because these subjects completed ≥11 CR sessions after randomization and prior to discharge. Among these 32 patients, the per session frequency of upward workload adjustment for subjects in the RPE, THRR, and THRR+HRM groups was 39, 45, and 60% (ηp2=.15, p=.008) from baseline to T1. Exercise HR did not increase significantly in the RPE group (1±6 and 2±6 bpm) from baseline to T1 and from T1 to T2, respectively (p=.56, p=.30). However, exercise HR did increase significantly from baseline to T1 in the THRR and THRR+HRM groups by 7±3 bpm and 12±2 bpm, respectively, (ηp2=.20, p=.021). Exercise HR increased significantly from T2 to T3 for subjects in the THRR group and was unchanged for subjects in the THRR+HRM group (4±1.5 bpm, ηp2 =.39, p=.009). The mean RPE was not significantly different at any time point, between groups at 2.9±.05, 3.1±.06, and 3.0±.05 in the RPE, THRR, THRR+HRM groups respectively (ηp2=.11, p=.19, Figure 4B).
The increases in exercise workload METs from baseline to end of CR were not significantly different between groups (1.5±1.0, 1.8±1.3, 2.4±1.0,ηp2 =.11, p=.20, for the RPE, THRR, and THRR+HRM groups, respectively). There was no statistically significant difference in peak VO2 or peak METS after CR among the 24 patients who completed the exit CPX (Table 2). However, the effect sizes for total treadmill time and VO2peak were medium to large (.02 and .19, respectively). VO2peak was highest in subjects in the THRR+HRM group (25±8 mL/kg/min, ηp2 =.18). Total treadmill time (10.6±2.2) measured during the CPX was highest in the THRR+HRM group (ηp2 =.02, p=.053). Patients in all groups strongly agreed with statements endorsing plans to continue exercise, program enjoyment, and understanding of prescription methods based on the results from patient satisfaction survey (eTable 1).
DISCUSSION
Although recruitment and retention were limited due to the COVID-19 pandemic, we were able to implement a prospective, open-label randomized controlled study implementing high fidelity THRR with or without the addition of a HRM in a previously all-RPE CR program. While demonstrating clear feasibility, we also found significant differences in exercise HR and the frequency of work-load adjustments in the THRR groups early in CR. We also found large effect sizes for changes in exercise training workload METS and exit exercise capacity between RPE, THRR, THRR+HRM groups. Finally, we found no differences in RPE ratings between the groups, suggesting that RPE may not be an ideal tool to use in CR when a THRR can be utilized using a recent a maximal exercise test.
Our findings highlight the critical importance of exercise intensity in CR. It is essential that CR professionals prescribe an exercise workload that is high enough to induce a training effect but not so high as to provoke abnormal clinical signs and symptoms.23–25 Subjects in the THRR and THRR+HRM groups gained an additional .7 and 1.3 workload METS compared to subjects in the RPE group, while not statistically significant, could be clinically significant.2,26 It is well known that a 1-MET increase in exercise capacity reduces subsequent risk of mortality by ~20%.2,5,27 The large effect size of .13 for exercise method on VO2peak provides evidence for the importance of exercise intensity during CR as improvements in CRF will reduce mortality risk and higher workloads will lead to greater health related benefits.2,5,24 Thus, the increase in workload METS and the large effect size for VO2peak in the THRR and THRR+HRM groups provides compelling preliminary evidence for the importance of maximal exercise testing and the use of objective measures to determine exercise intensity.
We originally included the THRR+HRM group to assure that patients were exercising within their assigned THRR, however, this was not necessary, as we saw no significant difference in the percent of HRR subjects were exercising in or in the number of sessions completed between the THRR and THRR+HRM groups. Therefore, the HRM did not improve fidelity of THRR within CR and is not needed during CR. However, wearable devices accompanied with exercise feedback have been shown to improve CRF and increase exercise duration.25 This could explain the non-significant but potentially clinically important increases in VO2peak and workload METS in the THRR+HRM group. Notably, home exercise and the use of the HRM outside of CR was not recorded, therefore, it is possible that subjects in the THRR+HRM group may have used the HRM to optimize their exercise intensity when exercising outside of CR.
Our study provides a cautionary tale for CR programs and clinicians that rely solely on RPE. Consistent with our findings, prior studies have shown that RPE may lead patients to underestimate a moderate exercise intensity as well as provide similar ratings when below, within, and above a THRR.26 27 Therefore, RPE alone may not be an sufficient tool for patients to guide the intensity of exercise (eFigure 1).29,32 If RPE is used, it should be used with high fidelity incorporating biophysical feedback, physiological anchors, and objectively measured whole body ratings recorded during exercise. Although RPE is easy to implement, it can result in inconsistencies in exercise intensity, particularly in elderly or obese patients.7 While THRR provides consistent intensities, a GXT is needed to measure peak HR and is not universally available or utilized.
There were several strengths to this study: it was a randomized trial, groups were well balanced at baseline, including baseline exercise workload, HR and workload adjustments were collected, there was good adherence to the protocol, satisfaction was high, and we observed no crossover between groups.
However, there were also limitations to this study. We were unable to measure changes in peak exercise capacity as well as home exercise. Baseline exercises tests were limited to the THRR groups to avoid group crossover as well as to examine the impact of fear and exercise self-efficacy as secondary outcomes reported in forthcoming manuscript. This limitation was minimized through randomization, examining workload METS, and peak VO2 at discharge from CR. Furthermore, exercise testing is rare and our RPE group is representative of most exercise prescription in the US.33 Statistical power was lower as this was a pilot study and there were COVID-19 related dropouts which impacted sample size and our ability to analyze the complete sample. This was a single center, open-label trial, therefore, it was not possible to blind staff from group assignment and secondary outcomes recorded during CR, but the stress lab staff that completed and interpreted the CPX were blind to group assignment.
CONCLSUION
Subjects prescribed exercise based on a THRR had more frequent upward adjustments in exercise workload early in CR, higher exercise HRs, and higher changes in HR from rest compared to exercise prescribed based upon RPE. Although, we noted large effect sizes for change in workload METS and VO2peak, these findings need to be confirmed in a large fully-powered trial.
Supplementary Material
Funding:
This study was funded by internal funds through Baystate Medical Center’s Researcher Pilot Award Program (RPAP), a K23 grant (1K23HL135440) from NHLBI awarded to Dr. Quinn Pack, and by RedCap (UL1RR025752, UL1TR000073, UL1TR001064, and UL1TR002544) through Tufts.
References
- 1.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brawner CA, Abdul-Nour K, Lewis B, et al. Relationship Between Exercise Workload During Cardiac Rehabilitation and Outcomes in Patients With Coronary Heart Disease. Am J Cardiol. 2016;117(8):1236–1241. [DOI] [PubMed] [Google Scholar]
- 3.Squires RW, Kaminsky LA, Porcari JP, Ruff JE, Savage PD, Williams MA. Progression of Exercise Training in Early Outpatient Cardiac Rehabilitation: AN OFFICIAL STATEMENT FROM THE AMERICAN ASSOCIATION OF CARDIOVASCULAR AND PULMONARY REHABILITATION. J Cardiopulm Rehabil Prev. 2018;38(3):139–146. [DOI] [PubMed] [Google Scholar]
- 4.Franklin BA, Lavie CJ, Squires RW, Milani RV. Exercise-based cardiac rehabilitation and improvements in cardiorespiratory fitness: implications regarding patient benefit. Mayo Clin Proc. 2013;88(5):431–437. [DOI] [PubMed] [Google Scholar]
- 5.Martin BJ, Arena R, Haykowsky M, et al. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc. 2013;88(5):455–463. [DOI] [PubMed] [Google Scholar]
- 6.Keteyian SJ, Brawner CA, Savage PD, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J. 2008;156(2):292–300. [DOI] [PubMed] [Google Scholar]
- 7.Rengo JL, Khadanga S, Savage PD, Ades PA. Response to Exercise Training During Cardiac Rehabilitation Differs by Sex. J Cardiopulm Rehabil Prev. 2020;40(5):319–324. [DOI] [PubMed] [Google Scholar]
- 8.Conraads VM, Denollet J, De Maeyer C, Van Craenenbroeck E, Verheyen J, Beckers P. Exercise training as an essential component of cardiac rehabilitation. Heart Br Card Soc. 2012;98(8):674–675; author reply 675. [DOI] [PubMed] [Google Scholar]
- 9.BACPR Elected Council Members. RAMIT presents an outdated version of cardiac rehabilitation. Heart Br Card Soc. 2012;98(8):672; author reply 673–674. [DOI] [PubMed] [Google Scholar]
- 10.Brawner CA, Al-Mallah MH, Ehrman JK, Qureshi WT, Blaha MJ, Keteyian SJ. Change in Maximal Exercise Capacity Is Associated With Survival in Men and Women. Mayo Clin Proc. 2017;92(3):383–390. [DOI] [PubMed] [Google Scholar]
- 11.Keteyian SJ, Kerrigan DJ, Ehrman JK, Brawner CA. Exercise Training Workloads Upon Exit From Cardiac Rehabilitation in Men and Women: THE HENRY FORD HOSPITAL EXPERIENCE. J Cardiopulm Rehabil Prev. 2017;37(4):257–261. [DOI] [PubMed] [Google Scholar]
- 12.Pack QR, Visintainer P, Farah M, et al. Development of a Simple Clinical Tool for Predicting Early Dropout in Cardiac Rehabilitation: A SINGLE-CENTER RISK MODEL. J Cardiopulm Rehabil Prev. 2020;Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180–191. [DOI] [PubMed] [Google Scholar]
- 14.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGill JB. Optimal use of beta-blockers in high-risk hypertension: a guide to dosing equivalence. Vasc Health Risk Manag. 2010;6:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pack QR, Bauldoff G, Lichtman SW, et al. Prioritization, Development, and Validation of American Association of Cardiovascular and Pulmonary Rehabilitation Performance Measures. J Cardiopulm Rehabil Prev. 2018;38(4):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekkekakis P. Let them roam free? Physiological and psychological evidence for the potential of self-selected exercise intensity in public health. Sports Med Auckl NZ. 2009;39(10):857–888. [DOI] [PubMed] [Google Scholar]
- 18.Williams DM, Dunsiger S, Miranda R, et al. Recommending self-paced exercise among overweight and obese adults: a randomized pilot study. Ann Behav Med Publ Soc Behav Med. 2015;49(2):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neil S, Thomas A, Pettit-Mee R, et al. Exercise Prescription Techniques in Cardiac Rehabilitation Centers in Midwest States. J Clin Exerc Physiol. 2018;7(1):8–14. [Google Scholar]
- 20.Mytinger M, Nelson RK, Zuhl M. Exercise Prescription Guidelines for Cardiovascular Disease Patients in the Absence of a Baseline Stress Test. J Cardiovasc Dev Dis. 2020;7(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas RJ, Balady G, Banka G, et al. 2018 ACC/AHA Clinical Performance and Quality Measures for Cardiac Rehabilitation: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circ Cardiovasc Qual Outcomes. 2018;11(4). [DOI] [PubMed] [Google Scholar]
- 22.Sydó N, Abdelmoneim SS, Mulvagh SL, Merkely B, Gulati M, Allison TG. Relationship Between Exercise Heart Rate and Age in Men vs Women. Mayo Clin Proc. 2014;89(12):1664–1672. [DOI] [PubMed] [Google Scholar]
- 23.Harber MP, Kaminsky LA, Arena R, et al. Impact of Cardiorespiratory Fitness on All-Cause and Disease-Specific Mortality: Advances Since 2009. Prog Cardiovasc Dis. 2017;60(1):11–20. [DOI] [PubMed] [Google Scholar]
- 24.Reed JL, Blais AZ, Keast ML, Pipe AL, Reid RD. Performance of Fixed Heart Rate Increment Targets of 20 vs 30 Beats per Minute for Exercise Rehabilitation Prescription in Outpatients With Heart Failure. Can J Cardiol. 2017;33(6):777–784. [DOI] [PubMed] [Google Scholar]
- 25.Joo KC, Brubaker PH, MacDougall A, Saikin AM, Ross JH, Whaley MH. Exercise prescription using resting heart rate plus 20 or perceived exertion in cardiac rehabilitation. J Cardpulm Rehabil. 2004;24(3):178–184; quiz 185–186. [DOI] [PubMed] [Google Scholar]
- 26.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. [DOI] [PubMed] [Google Scholar]
- 27.Ades PA, Savage PD, Brawner CA, et al. Aerobic capacity in patients entering cardiac rehabilitation. Circulation. 2006;113(23):2706–2712. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell BL, Lock MJ, Davison K, Parfitt G, Buckley JP, Eston RG. What is the effect of aerobic exercise intensity on cardiorespiratory fitness in those undergoing cardiac rehabilitation? A systematic review with meta-analysis. Br J Sports Med. 2019;53(21):1341–1351. [DOI] [PubMed] [Google Scholar]
- 29.Cadmus-Bertram LA, Marcus BH, Patterson RE, Parker BA, Morey BL. Randomized Trial of a Fitbit-Based Physical Activity Intervention for Women. Am J Prev Med. 2015;49(3):414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canning KL, Brown RE, Jamnik VK, Salmon A, Ardern CI, Kuk JL. Individuals Underestimate Moderate and Vigorous Intensity Physical Activity. Earnest CP, ed. PLoS ONE. 2014;9(5):e97927. doi: 10.1371/journal.pone.0097927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosiek RM, Szymanski LM, Lox CL, Kelley G, Macfarlane PA. Self-regulation of exercise intensity in cardiac rehabilitation participants. Sports Med Train Rehabil. 1998;8(4):359–368. [Google Scholar]
- 32.Gondoni LA, Nibbio F, Caetani G, Augello G, Titon AM. What are we measuring? Considerations on subjective ratings of perceived exertion in obese patients for exercise prescription in cardiac rehabilitation programs. Int J Cardiol. 2010;140(2):236–238. [DOI] [PubMed] [Google Scholar]
- 33.Pack Quinn R., Shea Meredith G., Brawner Clinton A., et al. Exercise Testing and Exercise Prescription in Cardiac Rehabilitation: A National Survey. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.