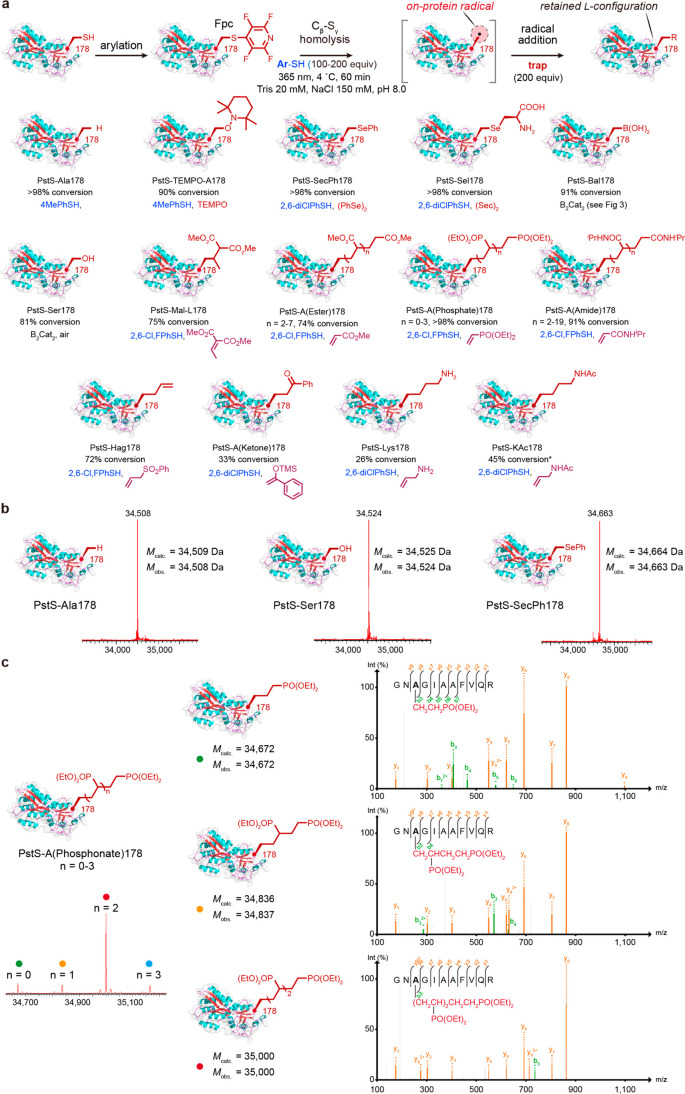
Figure 4.
Scope of l-alanyl radical-trapping. (a) Diverse bond-forming proves possible through trapping of the on-protein radical generated from PstS-Fpc178 to generate varied side chains. Please also see the Supplementary Methods where the acceptors used have been described in detail with individual schemes and structures for each. (b) Representative examples of Cβ–Hγ, Cβ–Oγ, and Cβ–Seγ bond formation proceed with excellent conversions. (c) Cβ–Cγ bond formation allows differing modes of bond formation. For example, on-protein C–C polymerization of vinyl phosphonate (which gives rise to a stabilized adduct radical product that can react further via matched polarity) can be observed via individual oligomer states using both intact protein MS (bottom left) and precisely mapped by tryptic-MSMS analyses (right). Direct Cβ–Cγ trapping without polymerization may also be achieved with differing substrates. In all cases, conversions are essentially full and the major side product formed in lower yielding reactions is the reduced Ala product. *: for KAc formation in PstS, the formation of KAc includes 40% “dimer” formation; interestingly, in histone H3 at site 18, only KAc product is observed (see Figure 5).