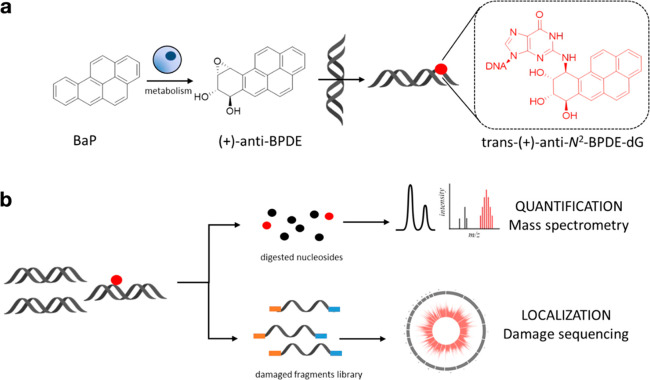
Figure 1.
(a) Benzo[a]pyrene (BaP) metabolism yields four stereoisomers of benzo[a]pyrene-diol-epoxide (BPDE): (+)-anti (shown), (−)-anti, (+)-syn, and (−)-syn. The metabolite reacts with DNA and undergoes trans- (shown) or cis-ring opening, leading to the formation of 8 potential isomers. The trans-(+)-anti-N2-BPDE-dG adduct shown is the most abundant and mutagenic. (b) Top: N2-BPDE-dG quantification workflow, involving enzymatic hydrolysis of genomic DNA, followed by chromatographic separation and quantification of deoxynucleosides by LC-MS/MS. In this study, cells were exposed to (±)-anti-BPDE, and total N2-BPDE-dG was quantified. Bottom: N2-BPDE-dG-sequencing workflow, involving denaturation and immunoprecipitation of genomic DNA, followed by marking of adduct sites by DNA extension synthesis with Q5 DNA polymerase. Amplified fragments are sequenced, and the DNA adducts are located at the −1 position relative to read starts.