Figure 7.
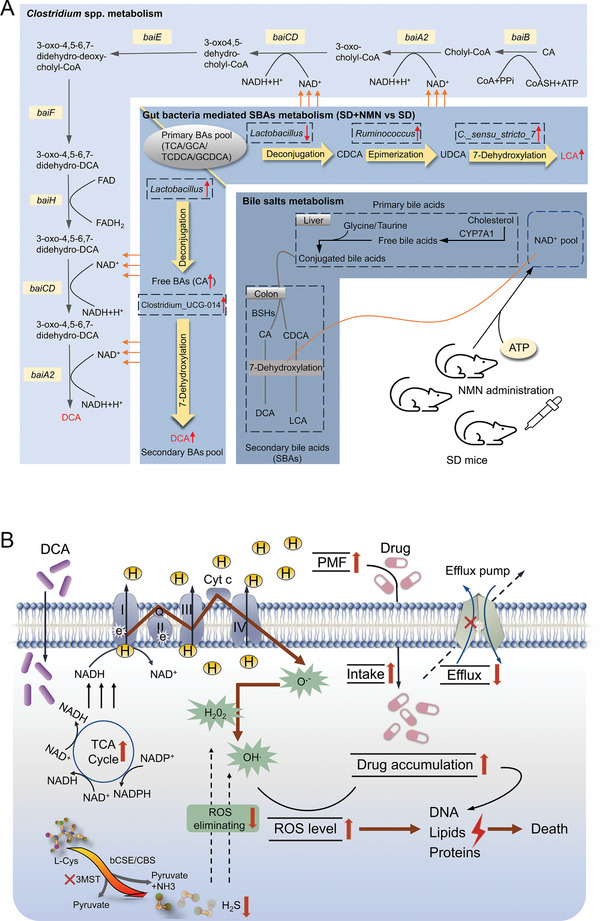
Schematic illustration of the proposed mechanisms for enhanced CR after NMN supplementation in SD mice and the antibacterial actions of DCA. A) The roles of NMN supplementation, gut microbiota, and bile acids metabolism in the maintenance of intestinal CR. In SD mice, decreased Lactobacillus reduces deconjugation of primary bile acids, while increased Ruminococcus and C._sensu_stricto_7 accelerate the epimerization of CDCA and the 7‐dehydroxylation of UDCA, thereby promoting the accumulation of LCA (the top right part of the middle layer). In SD + NMN mice, increased Lactobacillus and Clostridium_UCG‐014 promote the transformation of primary bile acids to CA and CA to DCA by deconjugation and 7‐dehydroxylation stepwisely, resulting in the increased DCA level (the lower left part of the middle layer). In terms of 7‐dehydroxylation activity in Clostridium spp., NMN could positively activate this process by generating NAD+. B) Exogenous DCA firstly activates TCA flux and triggers the ETC activity in a NADH‐dependent way, yielding large amounts of superoxide O2 •− and increasing PMF build‐up by enhanced proton flux. Then, increased PMF promotes drug uptake and impaired efflux pump reduces drug outflow, which aggravates oxidant stress to bacteria cells, and thus inhibits DNA synthesis and replication. Meanwhile, a key enzyme of H2S production, 3MST, was inhibited by DCA and CIP, resulting in the compromised ROS elimination efficiency mediated by H2S. Overall, exogenous DCA promotes antibiotic accumulation and disrupts the balance between oxidative stress and antioxidant defense system, thereby potentiating antibiotic bactericidal efficacy and facilitating bacterial death. CA, cholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; TCA, taurocholic acid; GCA, glycocholic acid; TCDCA, taurochenodeoxycholic acid; GCDCA, glycochenodeoxycholic acid; CDCA, chenodeoxycholic acid; UDCA, ursodeoxycholic acid; LCA, lithocholic acid; CYP7A1, cytochrome P450 family 7 subfamily A member 1; BSHs, bile acid hydrolases.
