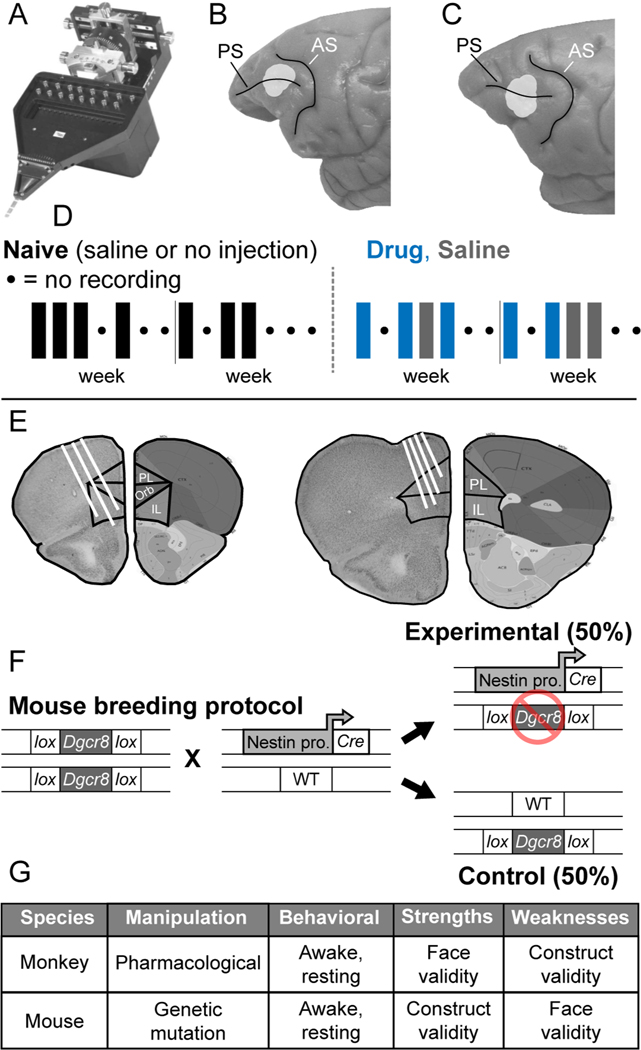
Figure 1. Experimental design.
(A) Spiking activity of small neural ensembles of individually isolated prefrontal neurons were recorded in monkeys and mice using an Eckhorn microelectrode drive (Thomas recording, GmbH) that advanced 16 thin (70 μm o.d.) glass coated platinum iridium microelectrodes independently into the brain under computer control. (B-C) Locations of ensemble neural recording (light gray shading) in Brodmann area 46 surrounding the principal sulcus (PS) in monkeys 1 (B) and 2 (C). (D) Neural recording sequence in relation to experimental conditions in monkeys. Neural activity was recorded in three conditions: (Naïve) before first exposure to NMDAR antagonist (either with no injection or after an injection of saline), (Drug) following intramuscular, systemic injection of NMDAR antagonist (phencyclidine, 0.25–0.30 mg/kg, i.m.), and (Saline) following intramuscular injection of saline but after first exposure to NMDAR antagonist. Once neural recording in the Naïve condition was completed, daily injections of either drug or saline with neural recording afterward commenced. For a complete description of the injection sequence, see47. (E) Electrode locations in coronal slices from two representative mouse brains. The right half of each slice is an image modified from the Allen Brain Atlas (Image credit: Allen Institute). The left half of each slice is a section through prefrontal cortex at the level of neural recording, with relevant border lines between prelimbic (PC) and infralimbic (IL) prefrontal areas superimposed, and recording tracts indicated (white lines). (F) Mouse breeding protocol. Nestin-Cre heterozygous and Dgcr8flox/flox homozygous mice were purchased from Jackson laboratories and interbred. Experimental offspring carried the Nestin-promoter and Cre gene and the Dgcr8flox allele, resulting in the deletion of a single Dgcr8 allele in neural tissue (referred to as Dgcr8+/−). Littermates with the Dgcr8flox allele but lacking Nestin-Cre were effectively wildtype (WT) and used as experimental controls. All genotypes were confirmed via standard tail snip genotyping protocols. (G) Complementary strengths and weaknesses of monkey drug and mouse genetic schizophrenia-relevant models112. The monkey drug model has comparatively strong face validity, because it captures cognitive behavioral deficits seen in patients with schizophrenia 67,113, but comparatively weak construct validity, because the schizophrenia-relevant manipulation is pharmacological. Conversely, the mouse genetic model has comparatively weak face validity, because of the large gap between the cognitive and behavioral capacities of mice and patients, but comparatively strong construct validity, because the schizophrenia-relevant manipulation relates to known genetic risk.