ABSTRACT
Individuals who are immunocompromised (IC) due to therapy or underlying disease are at increased risk of herpes zoster (HZ). This study evaluates the public health impact of recombinant zoster vaccine (RZV) relative to no HZ vaccination for the prevention of HZ among adults aged ≥18 years diagnosed with selected cancers in the United States (US). A static Markov model was used to simulate three cohorts of individuals who are IC with cancer (time horizon of 30 years; one-year cycle length): hematopoietic stem cell transplant (HSCT) recipients, patients with breast cancer (BC; a solid tumor example), and patients with Hodgkin’s lymphoma (HL; a hematological malignancy example). Cohort sizes reflect the estimated annual incidence of each condition in the US population (19,671 HSCT recipients, 279,100 patients with BC, and 8,480 patients with HL). Vaccination with RZV resulted in 2,297; 38,068; and 848 fewer HZ cases for HSCT recipients, patients with BC, and patients with HL, respectively (each versus no vaccine). Vaccination with RZV also resulted in 422; 3,184; and 93 fewer postherpetic neuralgia cases for HSCT, BC, and HL, respectively. Analyses estimated the quality-adjusted life years gained to be 109, 506, and 17 for HSCT, BC, and HL, respectively. To prevent one HZ case, the number needed to vaccinate was 9, 8, and 10, for HSCT, BC, and HL, respectively. These results suggest RZV vaccination may be an effective option to significantly reduce HZ disease burden among patients diagnosed with selected cancers in the US.
KEYWORDS: Herpes zoster, herpes zoster vaccine, hematopoietic stem cell transplantation, neoplasms, breast neoplasms, Hodgkin disease, public health
Plain Language Summary
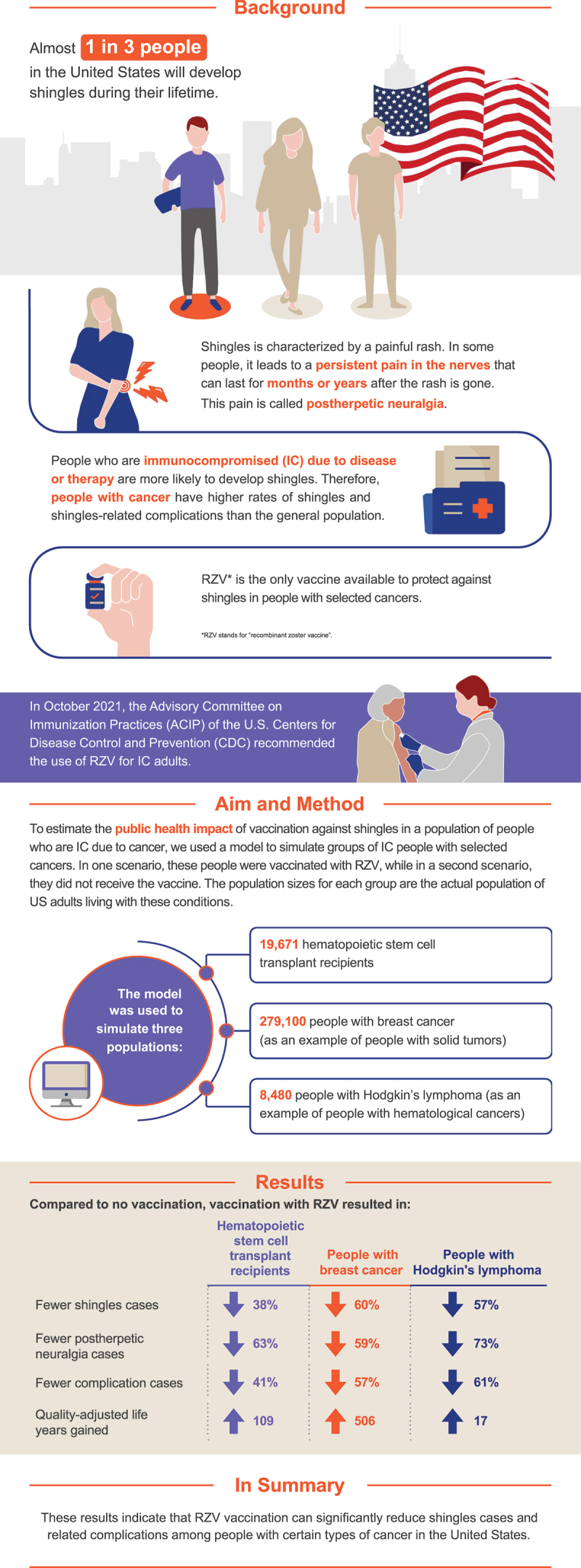
Shingles cases can be prevented by recombinant zoster vaccine (RZV). People who have a weakened immune system (immunocompromised) due to disease or therapy are more likely to develop shingles. For example, shingles occurs in nearly a quarter of patients receiving immunosuppressive treatment for blood cancers. To estimate the public health impact of vaccination against shingles in people who are immunocompromised due to cancer in the United States (US), we used a model to simulate groups with selected types of cancer. The results indicate vaccination with RZV can significantly reduce shingles cases and related complications among these groups in the US.
Introduction
Almost one in three individuals in the United States (US) will develop herpes zoster (HZ; also referred to as “shingles”) during their lifetime, resulting in approximately 1 million cases each year.1 HZ, caused by the reactivation of the varicella zoster virus (VZV), is characterized by a painful, unilateral rash that typically presents in one or two adjacent dermatomes. Many patients also experience HZ-related complications such as postherpetic neuralgia (PHN), a persistent pain that can last for months or years after the acute HZ rash has resolved. Although PHN is the most common complication of HZ, patients also may experience ocular, neurological, cutaneous, and/or other non-pain complications.1
Incidence of HZ in the general population gradually increases as individuals age due to immunosenescence.2 However, incidence increases sharply in populations who are immunocompromised (IC) due to known disease or therapy.3 The risk of HZ is highest among individuals receiving hematopoietic stem cell transplant (HSCT), which is often used to treat myeloma and lymphomas, among other diseases.3,4 Empirical evidence has also shown patients with either hematological malignancies or solid tumors are at increased risk of HZ and HZ-related complications due to immunosuppression.5–7 Patients with hematological cancers receiving immunosuppressive treatments are at especially high risk, with HZ occurring in up to a quarter of patients with multiple myeloma, Hodgkin’s lymphoma, and chronic lymphocytic leukemia receiving such treatments.8
In addition to the higher risk of HZ, populations who are IC have a higher risk of developing persistent PHN and a higher disease burden, given a case of HZ.4,9,10 As an example, patients diagnosed with cancer have higher rates of HZ-related complications than the general population.6 Individuals with an impaired immune status also had HZ severity of illness scores twice as high as individuals with normal immune function, and duration of HZ has been shown to be longer for HSCT recipients, compared to older adults.11,12 These results align with a study in a US adult community population that found that although 8% of HZ cases occurred among individuals who are IC, these individuals represented 23.8% of the total HZ-related costs.13
Pivotal trials indicate recombinant zoster vaccine (RZV) is safe and elicits immunogenicity when administered to adults with hematological cancers receiving immunosuppressive treatments or with solid tumors before or at the start of a chemotherapy cycle.8,14 A pivotal phase 3 clinical trial of autologous HSCT recipients who received RZV found it had 68.2% vaccine efficacy against HZ and significantly reduced HZ-related complications.15 Additionally, it was demonstrated that even when RZV failed to prevent HZ, vaccine recipients experienced less severe pain associated with HZ. Consequently, higher vaccine efficacy was observed in preventing PHN (i.e., efficacy of 89.3%), and in reducing the burden of illness associated with HZ-related pain (i.e., 82.5%) compared to vaccine efficacy against HZ (i.e., 68.2%). The study also demonstrated that vaccination with RZV significantly reduced the impact of HZ on social functioning, role emotional, and mental health scores in patients who developed HZ despite vaccination.12
Since October 2017, RZV has been recommended for prevention of HZ in adults aged ≥50 years.16 In July 2021, the U.S. Food and Drug Administration (FDA) expanded the indication for RZV to include adults aged ≥18 years who are or will be at increased risk for HZ because of immunodeficiency or immunosuppression caused by known disease or therapy. In October 2021, the Advisory Committee on Immunization Practices (ACIP) recommended RZV for adults aged ≥19 years who are or will be at increased risk for HZ because of immunodeficiency or immunosuppression caused by known disease or therapy, aligning closely with the updated FDA indication for these patients aged 18 and older.17,18
Previous research has demonstrated the public health impact of RZV for the prevention of HZ in immunocompetent (“healthy” hereafter) adults aged ≥50 years reduced HZ disease burden.19 However, to our knowledge, the public health impact of RZV in adults with cancer has not yet been comprehensively evaluated. The objective of the current study was to assess the public health impact of RZV versus no vaccine for the prevention of HZ among adults who are IC aged ≥18 years diagnosed with selected cancers in the US.
A graphical plain language summary of the study is provided in Figure 1.
Figure 1.
Plain language summary.
Methods
Model overview
An illustration of the ZOster ecoNomic Analysis (ZONA) IC model structure and analysis framework is provided in Figure 2. The ZONA IC Model was programmed in Microsoft Excel, and was adapted from the ZONA 50+ Model, which focused on populations aged 50 years and older.20,21 The ZONA IC Model observes HZ status and outcomes in patients who are IC at the start of the model’s time horizon and the maintain an IC status for a defined number of years (the “IC status duration”). After the IC status duration, individuals transition to the healthy status and remain with that status for the remaining years of the time horizon. The underlying structure is a deterministic, single-cohort, static Markov model with a cycle length of 1 year.
Figure 2.
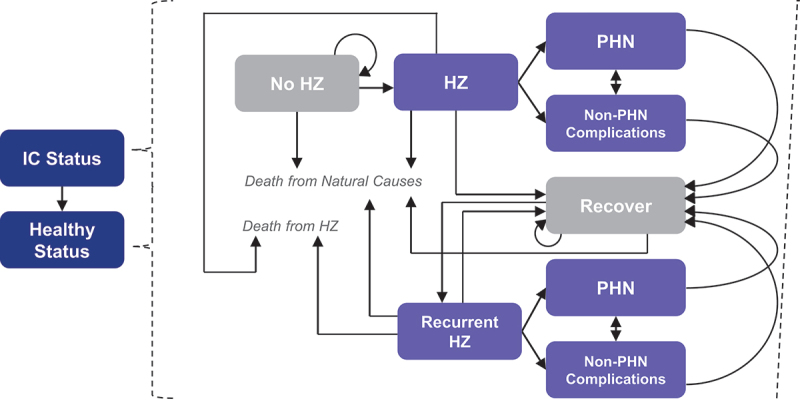
ZONA IC model structure and analysis framework.
HZ = herpes zoster; IC = immunocompromised; PHN = postherpetic neuralgia; ZONA = ZOster ecoNomic Analysis.
Vaccination may or may not occur in the no HZ health state, depending on the modeled strategy.
In the ZONA IC Model, outcomes are observed for the modeled population under each of two intervention strategies: RZV and No Vaccine. For the RZV strategy, vaccination of the entire population occurs at the simulation start. For the No Vaccine strategy, vaccination does not occur. Individuals who experience HZ incur reductions in their quality of life (reflected as reduced quality-adjusted life years [QALYs]) and may experience complications. The HZ complications included in the model are PHN and non-PHN complications, including ocular, neurological, cutaneous, and other non-pain complications. As shown in Figure 2, HZ with PHN and HZ with other complications are separate health states in the model. Recurrent HZ and its associated health states are distinct from those for patients with their first occurrence of HZ.
The ZONA IC model was used to simulate three cohorts of individuals for a period of 30 years: (1) HSCT recipients, (2) patients with breast cancer, and (3) patients with Hodgkin’s lymphoma. The three conditions selected were ones in which RZV had been evaluated in randomized clinical trials.8,14,15 The cohort sizes were based on the estimated size of the US population diagnosed with these conditions each year. The number of HSCT recipients was equal to the total number of HSCTs performed on individuals aged ≥21 years in 2017 in the US (19,671).22 The numbers of patients with breast cancer (279,100) and Hodgkin’s lymphoma (8,480) were both equal to the total number of individuals of all ages projected to be diagnosed with each respective condition in 2020.23 As these incidence estimates were not available based on age, the results of this model assume all new cases are among adults aged ≥18 years. The model used a one-year cycle length and compared HZ-related health outcomes for vaccination with RZV versus no HZ vaccination.
Patients with breast cancer were modeled as an example of the population of patients with solid tumors, and patients with Hodgkin’s lymphoma were modeled as an example of the population of patients with hematological cancers. Since there were no vaccine efficacy data available from clinical trials for the modeled populations with breast cancer and with Hodgkin’s lymphoma, regression analyses were conducted based on the association between HZ incidence estimates in placebo arms of RZV clinical trials and efficacy and waning estimates for healthy and IC populations in the trials.8,15,24,25 The regression analyses estimated potential differences in RZV efficacy based on the magnitude of HZ risk during IC status, and were applied to the various annual HZ incidence values observed in the analyses.
Model inputs
Model input values were identified through a targeted literature review focused on publications from 2005 to 2020. Input values for RZV efficacy and waning were based on pivotal clinical trial data, with the analysis assuming 100% 2-dose compliance for consistency with a previous economic analysis of HZ vaccination.15,26 Epidemiological and mortality inputs were obtained from published literature and national survival statistics, respectively. Tables S1 and S2 provide epidemiological and vaccine-specific model input values used in the base-case and sensitivity analyses.
There were two sets of model inputs: one set that was applied while the modeled population was IC due to disease or therapy (“IC status”) and a second set that was applied when the modeled population recovered immune function (“healthy status”). Whichever input applied depended on whether the individual was still within the IC status transient window; the duration of the IC state differed between the modeled populations. For the population of HSCT recipients, a five-year duration of IC status was chosen based on a study that found the incidence rate of HZ in HSCT recipients over five years of follow up (60 per 1,000 person-years) was approximately ten times higher than the reported incidence rate in healthy adults (4 per 1,000 person-years).27 For both patients with breast cancer and patients with Hodgkin’s lymphoma, a two-year duration of IC status was used, based on a study that reported the annual incidence of HZ in patients with hematologic malignancies, solid tumor malignancies, and by level of immunosuppression.6 Different all-cause mortality rates were used for the healthy (immunocompetent) and IC populations. For the modeled population of HSCT recipients, a probabilistic sensitivity analysis (PSA) was conducted to explore the uncertainty around the model’s outcomes, given the uncertainty around the model’s inputs.
Some model inputs have values that vary by age. Individuals were stratified into six age groups: 18–49; 50–59; 60–64; 65–69; 70–79; and ≥80. As a cohort ages annually over the modeled time horizon, the inputs corresponding to the cohort’s age are applied (Table S1). For example, if the starting age of the cohort is 35 years, then the value for case fatality rate for HZ cases for the cohort aged 35 to 49 years is applied for the first 15 years of the model (while the cohort is between 35 and 49 years old); in years 16 to 20 of the analysis, the value for the cohort aged 50 to 54 is applied, and so on. Only those values applied to individuals with IC status are displayed in Tables S1 and S2. The values applied to individuals with healthy status have been previously described.20
The population starting age varied for the base-case analyses. In the population of HSCT recipients, the selected starting age was 35 years to represent a younger HSCT population (i.e., approximately the midpoint of ages 18 to 49 years), although 75% of the population in a pivotal clinical trial reporting RZV efficacy in the HSCT population were aged ≥50 years.15 In the breast cancer population analysis, the starting age of the population was 45 years to represent a younger breast cancer population and was based on National Cancer Institute data, which reported 18.8% of breast cancer diagnoses occurred in patients between the ages of 45 and 54 years (Table S2).28 In the Hodgkin’s lymphoma population scenario, the starting age was assumed to be 25, based on National Cancer Institute data, which reported Hodgkin’s lymphoma was most frequently diagnosed among adults aged 20 to 34 years.29
Results
Table 1 and Figure 3 present results of the analyses of the three IC populations.
Table 1.
Results for selected cancer populations vaccinated with RZV versus no vaccine.
| Outcomes for RZV versus no vaccine | HSCT | Breast cancer | Hodgkin’s lymphoma |
|---|---|---|---|
| Cases avoided during 30-year time horizon | |||
| HZ cases | 2,297 | 38,068 | 848 |
| PHN cases | 422 | 3,184 | 93 |
| Complication cases | 271 | 3,812 | 80 |
| Ocular | 81 | 1,360 | 28 |
| Neurological | 41 | 1,024 | 14 |
| Cutaneous | 129 | 905 | 30 |
| Other non-pain | 20 | 521 | 7 |
| Cases avoided during IC period | |||
| HZ cases | 2,210 | 8,435 | 438 |
| PHN cases | 403 | 1,095 | 63 |
| Complication cases | 265 | 1,012 | 52 |
| Ocular | 78 | 300 | 15 |
| Neurological | 39 | 149 | 7 |
| Cutaneous | 127 | 487 | 25 |
| Other non-pain | 19 | 75 | 3 |
| QALYs gained | 109 | 506 | 17 |
HSCT = hematopoietic stem cell transplant; HZ = herpes zoster; IC = immunocompromised; PHN = postherpetic neuralgia; QALYs = quality-adjusted life years; RZV = recombinant zoster vaccine.
Figure 3.
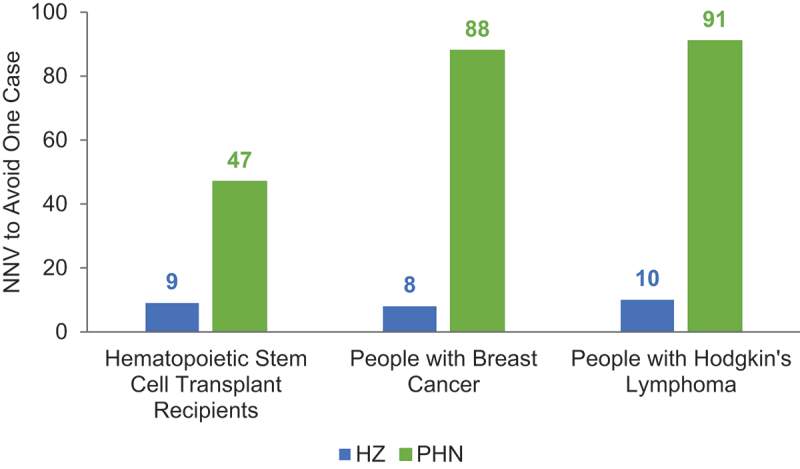
Number needed to vaccinate to avoid one HZ case and one PHN case, by population.
HSCT = hematopoietic stem cell transplant; HZ = herpes zoster; IC = immunocompromised; NNV = number needed to vaccinate; PHN = postherpetic neuralgia.
In the modeled cohort of 19,671 autologous HSCT recipients over 30 years, vaccination with RZV resulted in 2,297 avoided cases of HZ (38% decrease); 422 avoided cases of PHN (63% decrease); 271 avoided cases of non-PHN complications (41% decrease); and 109 QALYs gained, due to decreased HZ morbidity. The number needed to vaccinate (NNV) to prevent one HZ case was 9, and the NNV to prevent one PHN case was 47. The results of the PSA for the modeled population of HSCT recipients are summarized in the histogram presented in Figure 4. For the overall 5,000 simulations, the number of HZ cases avoided with RZV versus no vaccination for HSCT recipients ranged from 400 to 4,000 extra cases.
Figure 4.
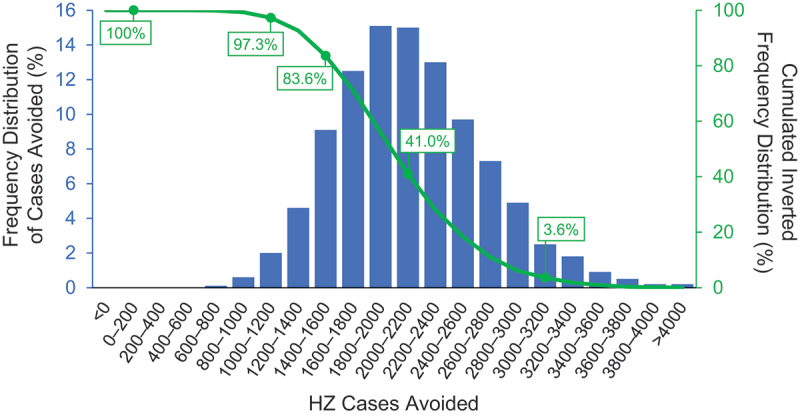
Probabilistic sensitivity analysis for HSCT population.
HSCT = hematopoietic stem cell transplant; HZ = herpes zoster.
Vaccination with RZV among individuals diagnosed with breast cancer resulted in 38,068 fewer cases of HZ (60% decrease); 3,184 cases avoided of PHN (59% decrease); 3,812 cases avoided of non-PHN complications (57% decrease); and 506 QALYs gained. The NNV to avoid one HZ or PHN case was 8 and 88, respectively. Among individuals diagnosed with Hodgkin’s lymphoma, vaccination with RZV resulted in 848 fewer HZ cases (57% decrease); 93 avoided cases of PHN (73% decrease); 80 cases avoided of non-PHN complications (61% decrease); and 17 QALYs gained. The NNV to avoid one HZ or PHN case was 10 and 91, respectively.
Discussion
Patients with cancer are significantly IC with a high risk of HZ and high disease burden. RZV is the first vaccine licensed and recommended to prevent HZ in IC populations.30 Hence, it is important to demonstrate the public health impact of using RZV in IC populations with selected cancers to inform health care decisions. Our results are consistent with the study performed by the US Centers for Disease Control and Prevention (CDC), the results of which informed the 2021 ACIP recommendation for vaccination with RZV in adults aged ≥19 years who are or will be at increased risk for HZ because of immunodeficiency or immunosuppression caused by known disease or therapy. In particular, the CDC analysis found that in a population of adult HSCT recipients, the NNV to avoid one HZ or PHN case was similar to those reported in the present study.31 Although there are no studies other than the CDC model to compare against, we modeled three different cancer populations to provide sufficient insights to allow for a sound interpretation of results in the populations studied.
It is important to note that the immunocompromised population is a heterogenous population with knowledge gaps. Hence, we identified “proxy” conditions where data are readily available, driven by clinical trials, published literature around disease burden and dialogue with the CDC’s Advisory Committee on Immunization Practices (ACIP). We included HSCT recipients because they represent a cancer population with the highest HZ risk,4 and the efficacy of RZV has been estimated in this population.15 RZV has also been found to be immunogenic and safe in patients with hematological and solid tumors, and the epidemiology of HZ in patients with cancer is well studied.8,14 Patients with breast cancer and patients with Hodgkin’s lymphoma were chosen to represent patients with solid tumors and hematological malignancies, respectively.6 Further clinical trials and studies to assess RZV and to increase the body of knowledge in immunocompromised populations are ongoing.
As expected in the HSCT cohort, the cases avoided during the 30-year time horizon were very similar to the cases avoided during the IC period. For the modeled HSCT cohort, the “IC status duration” was set to 5 years. Hence, we applied the immunocompromised values of HZ incidence (60 per 1,000) for the first 5 years, which are substantially higher than the values for the healthy population (3–12 per 1,000) that were used from years 6–30. Therefore, for the first 5 years of the analysis, the high incidence rate was applied, until the patients transitioned to the healthy status at year 6. For years 6–30, the HZ risk dropped back to the healthy level, thus accruing fewer HZ cases. There are also fewer cases avoided during years 6–30 because vaccine efficacy wanes quickly in HSCT recipients (9.1%; Table S2). Therefore, these two factors (incidence and efficacy) explain why the majority of HZ cases avoided for the HSCT cohort were observed during the first 5 years of the analysis.
There is potentially a significant additional public health impact in the populations with solid tumors and hematological malignancies beyond that estimated in this study, since the present study only evaluated subpopulations of these cancer types. The prevalence of HZ in patients with selected cancers and the effectiveness of RZV in these populations underscores the importance of our study. RZV did not only protect patients from HZ, but also for those experiencing HZ, RZV reduced the burden of illness associated with HZ-related pain and significantly reduced the impact of HZ on social functioning, emotional, and mental health scores. This highlights the net positive impact of RZV beyond preventing HZ cases and stresses the importance of vaccination against HZ in improving the population’s quality of life.
The ACIP determined HZ in adults who are IC is of public health importance and recently unanimously recommended RZV for the prevention of HZ and related complications in adults aged ≥19 years who are or will be immunodeficient or immunosuppressed because of known disease or therapy.18 Our study illustrates the importance of vaccination with RZV for patients with selected cancers because of the high risk of HZ, with results indicating vaccination with RZV is an effective option to prevent HZ cases and reduce the HZ disease burden among patients diagnosed with selected cancers in the US.
Limitations
Decisions about the modeling of vaccine data were based on clinical trial results and data from a targeted literature review that was conducted to ascertain realistic input values. Our analysis made the simplifying assumption that the IC status duration was a discrete number of years, and that the population maintained a healthy status for the remainder of the time horizon. The IC duration and resulting HZ risk may be more dynamic in real-world IC populations. Furthermore, 100% second-dose compliance was assumed based on high compliance rates in RZV clinical trial populations, which may overestimate compliance in a real-world setting. However, given cancer patients have frequent interactions with health care providers due to their underlying conditions, we anticipate that high second-dose compliance is realistic. Additionally, the starting ages of the modeled populations were chosen to best represent cases potentially avoided under the updated ACIP recommendation for adults aged ≥19, focusing on younger adults (i.e., younger than 50 years of age) who are or will be at increased risk for HZ because of immunodeficiency or immunosuppression caused by known disease or therapy. As the mean ages of the populations in the real world are typically higher than the modeled starting ages, the model may not capture the full age-related HZ risk after the IC duration, and therefore may underestimate the true public health impact.
While previous studies have only reported HZ risk among patients with cancer up to two years after diagnosis,5,6 our analysis assumed HSCT patients were IC for 5 years based on an epidemiological study of HZ among autologous HSCT recipients.27 There were limited data available to estimate RZV effectiveness and durability in cancer populations other than HSCT recipients, so regression analyses were conducted using data from RZV clinical trials in healthy and IC populations to estimate these values. However, other immunological factors may contribute to differences in RZV effectiveness and durability across different cancer populations. Additionally, it should still be noted that the modeled populations only represent a subset of the wider cancer population.
Finally, efficacy estimates for the HSCT population were based on a clinical trial in which patients with more than 6 months of expected antiviral prophylaxis post-HSCT were excluded.15 However, Sahoo et al.27 found that patients taking antiviral prophylaxis had a lower risk of HZ versus those not taking prophylaxis. Further, Zhang et al.32 found that duration of antiviral prophylaxis greater than 6 months was associated with lower risk of HZ compared to duration of antiviral prophylaxis less than 3 months. Therefore, efficacy estimates in the study may be higher than in the real world where antiviral prophylaxis post-HSCT may be expected to continue beyond 6 months. In addition, immunosuppressive treatments may also impact the efficacy of the vaccine. As such, the CDC’s Morbidity and Mortality Weekly Report (MMWR) noted that, where possible, patients should be vaccinated before becoming immunosuppressed. It was also noted that providers should consider timing vaccination when the immune response is likely to be most robust.18 Additional guidelines on vaccination against herpes zoster in cancer populations are provided elsewhere.33–35
Conclusions
Our estimates show that RZV vaccination will prevent HZ cases and related complications among patients with selected cancers, and in turn significantly reduce the disease burden. These findings support the ACIP’s recommendation for RZV for the prevention of HZ and related complications in adults, specifically those who are or will be immunocompromised due to selected underlying cancers or cancer treatment.
Supplementary Material
Acknowledgments
The authors thank the investigators and their teams who took part in this study. The authors also thank Seri Anderson from RTI Health Solutions for providing assistance with the literature review, and documentation of sources and assumptions for the model inputs. The authors also thank Costello Medical for editorial assistance and publication coordination, on behalf of GSK, and acknowledge Isabel Katz from Costello Medical, USA for medical writing and editorial assistance based on the authors’ input and direction. This study was funded by GlaxoSmithKline Biologicals SA.
Funding Statement
This study was sponsored by GlaxoSmithKline Biologicals SA (HO-19-19750). Support for third-party writing assistance for this article was funded by GlaxoSmithKline Biologicals SA in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Authors’ contributions
Substantial contributions to study conception and design: DC, BJP, JC, AS, SL, KAH, CFC; substantial contributions to analysis and interpretation of the data: DC, BJP, JC, AS, EML, SL, KAH, SP, CFC; drafting the article or revising it critically for important intellectual content: DC, BJP, JC, AS, EML, SL, KAH, SP, CFC; final approval of the version of the article to be published: DC, BJP, JC, AS, EML, SL, KAH, SP, CFC.
Disclosure statement
DC, AS, EML and SP are employees of the GSK group of companies. DC, AS, EML and SP report holding shares in the GSK group of companies. JC and KAH report payments to RTI Health Solutions from the GSK group of companies for the conduct of this study. BJP reports holding shares in the GSK group of companies and is a former employee of the GSK group of companies. SL and CFC declare to have received consulting fees from the GSK group of companies. All authors declare no other financial and non-financial relationships and activities.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2167907
References
- 1.CDC . Shingles (herpes zoster): clinical overview; 2020.
- 2.Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ.. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15(1):1. doi: 10.1186/s12879-015-1262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S-Y, Suaya JA, Li Q, Galindo CM, Misurski D, Burstin S, Levin MJ. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42(2):325–8. doi: 10.1007/s15010-013-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKay SL, Guo A, Pergam SA, Dooling K. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125–34. doi: 10.1093/cid/ciz1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian J, Heywood AE, Karki S, Banks E, Macartney K, Chantrill L, Liu B. Risk of herpes zoster prior to and following cancer diagnosis and treatment: a population-based prospective cohort study. J Infect Dis. 2019;220:3–11. doi: 10.1093/infdis/jiy625. [DOI] [PubMed] [Google Scholar]
- 6.Habel LA, Ray GT, Silverberg MJ, Horberg MA, Yawn BP, Castillo AL, Quesenberry CP, Li Y, Sadier P, Tran TN. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(1):82–90. doi: 10.1158/1055-9965.EPI-12-0815. [DOI] [PubMed] [Google Scholar]
- 7.Hansson E, Forbes HJ, Langan SM, Smeeth L, Bhaskaran K. Herpes zoster risk after 21 specific cancers: population-based case–control study. Br J Cancer. 2017;116(12):1643–51. doi: 10.1038/bjc.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagnew AF, Ilhan O, Lee WS, Woszczyk D, Kwak J-Y, Bowcock S, Sohn SK, Rodriguez Macías G, Chiou T-J, Quiel D, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000. doi: 10.1016/S1473-3099(19)30163-X. [DOI] [PubMed] [Google Scholar]
- 9.Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, Langan SM. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157(1):30–54. doi: 10.1097/j.pain.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–21. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Drolet M, Brisson M, Schmader K, Levin M, Johnson R, Oxman M, Patrick D, Camden S, Mansi JA. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain. 2010;11(11):1211–21. doi: 10.1016/j.jpain.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Curran D, Matthews S, Rowley SD, Young JAH, Bastidas A, Anagnostopoulos A, Barista I, Chandrasekar PH, Dickinson M, El Idrissi M, et al. Recombinant zoster vaccine significantly reduces the impact on quality of life caused by herpes zoster in adult autologous hematopoietic stem cell transplant recipients: a randomized placebo-controlled trial (ZOE-HSCT). Biol Blood Marrow Transplant. 2019a;25(12):2474–81. doi: 10.1016/j.bbmt.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc. 2009;84(9):787–94. doi: 10.4065/84.9.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vink P, Mingorance ID, Alonso CM, Rubio-viqueira B, Jung KH, Rodriguez Moreno JF, Grande E, Marrupe Gonzalez D, Lowndes S, Puente J, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125(8):1301–12. doi: 10.1002/cncr.31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastidas A, de la Serna J, El Idrissi M, Oostvogels L, Quittet P, López-Jiménez J, Vural F, Pohlreich D, Zuckerman T, Issa NC, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. Jama. 2019;322(2):123–33. doi: 10.1001/jama.2019.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–08. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shingrix [package insert], revised: 07/2021. In: Administration FaD, editor. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. [Google Scholar]
- 18.Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM, Kotton CN, Dooling KL. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–84. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson BJ, Buck PO, Curran D, Van Oorschot D, Carrico J, Herring WL, Zhang Y, Stoddard JJ. Estimated public health impact of the recombinant zoster vaccine. Mayo Clin Proc Innov Qual Outcomes. 2021;5(3):596–604. doi: 10.1016/j.mayocpiqo.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran D, Patterson B, Varghese L, Van Oorschot D, Buck P, Carrico J, Hicks K, Lee B, Yawn B. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–45. doi: 10.1016/j.vaccine.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Curran D, Patterson BJ, Van Oorschot D, Buck PO, Carrico J, Hicks KA, Lee B, Yawn BP. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States who have been previously vaccinated with zoster vaccine live. Hum Vaccin Immunother. 2019b;15(4):765–71. doi: 10.1080/21645515.2018.1558689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Center for International Blood and Marrow Transplant Research . Transplant activity report covering 2013-2017. 2019.
- 23.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang S-J, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 25.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 26.Prosser LA, Harpaz R, Rose AM, Gebremariam A, Guo A, Ortega-Sanchez IR, Zhou F, Dooling K. A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Ann Intern Med. 2019;170(6):380–88. doi: 10.7326/M18-2347. [DOI] [PubMed] [Google Scholar]
- 27.Sahoo F, Hill JA, Xie H, Leisenring W, Yi J, Goyal S, Kimball LE, Lee I, Seo S, Davis C, et al. Herpes zoster in autologous hematopoietic cell transplant recipients in the era of acyclovir or valacyclovir prophylaxis and novel treatment and maintenance therapies. Biol Blood Marrow Transplant. 2017;23(3):505–11. doi: 10.1016/j.bbmt.2016.12.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noone AM, Howlander N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, et al. SEER cancer statistics review, 1975-2015. National Cancer Institute; 2018. [Google Scholar]
- 29.National Cancer Institute . Cancer stat facts: Hodgkin lymphoma; 2019.
- 30.The Advisory Committee on Immunization Practices . Altered immunocompetence. General best practice guidelines for immunization: best practices guidance of the Advisory Committee on Immunization Practices (ACIP): United States Government. 2022.
- 31.Leidner AJ, Anderson TC, Hong K, Ortega-Sanchez IR, Guo A, Pike J, Prosser LA, Dooling KL. Cost-effectiveness analysis of vaccination with recombinant zoster vaccine among hematopoietic cell transplant recipients and persons with other immunocompromising conditions aged 19 to 49 years. Value Health. 2022;S1098-3015(22):02143–X. doi: 10.1016/j.jval.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D, Weiss T, Feng Y, Finelli L. Duration of antiviral prophylaxis and risk of herpes zoster among patients receiving autologous hematopoietic stem cell transplants: a retrospective, observational study. Adv Ther. 2017. Jul;34(7):1610–21. doi: 10.1007/s12325-017-0553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D. Management of immunotherapy-related toxicities, Version 1. J Natl Compr Canc Netw. 2019;17(3):255–89. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 34.Jorga A, Friedland LR, Lecrenier N, Safonova E, Vink P, Widenmaier R. BPI22-019: guidelines and recommendations for the adjuvanted recombinant zoster vaccine in immunocompromised cancer patients. J Natl Compr Canc Netw. 2022;20(3.5):BPI22–019. doi: 10.6004/jnccn.2021.7194. [DOI] [Google Scholar]
- 35.Parikh R, Widenmaier R, Lecrenier N. A practitioner’s guide to the recombinant zoster vaccine: review of national vaccination recommendations. Expert Rev Vaccines. 2021;20(9):1065–75. doi: 10.1080/14760584.2021.1956906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


