Figure 4: Hydrazinolysis maps the order of ester crosslink formation in pre-amycolimiditide.
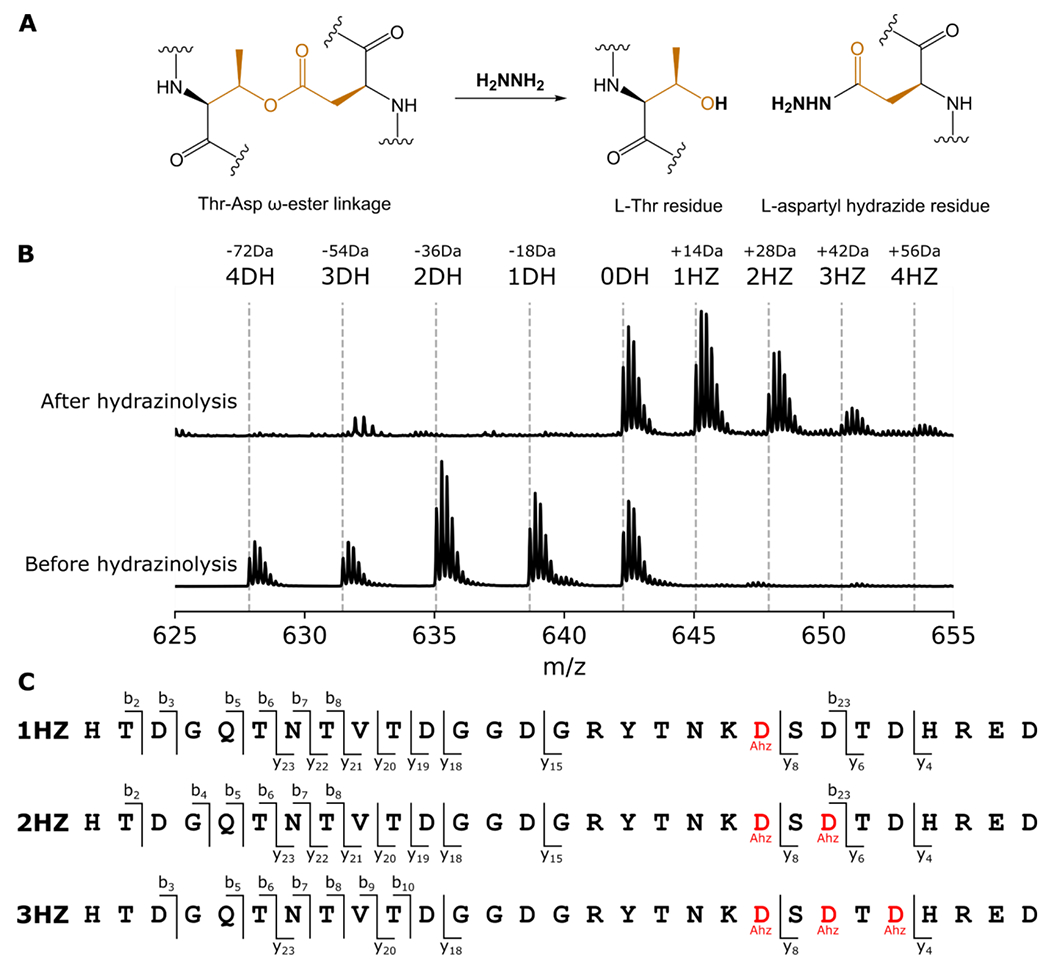
A: Mechanism of ester scission by hydrazine. The L-Thr residue is restored while the Asp residue is marked with a hydrazide. B: A mixture of intermediates of pre-amycolimiditide was generated in vitro and reacted with hydrazine. The mass spectrum shows a distribution of species in different dehydration states (bottom) and quantitative conversion to the hydrazide form (top). Note that the unmodified species (0DH) does not react with hydrazine, showing that the chemistry is specific toward ester linkages. C: Tandem mass spectrometry analysis of the hydrazine-labeled peptide mixture from part B. Hydrazide-labeled Asp residues are denoted as Ahz. In the singly labeled species (1HZ) only Asp21 is labeled with hydrazine whereas the doubly labeled species (2HZ) has Asp21 and Asp 23 labeled. The triply labeled 3HZ species is labeled at Asp21, Asp23, and Asp25, showing a specific order of ester formation. Mass spectra underlying this data are present in Fig. S29.
