Figure 6: Ester and aspartimide formation in disulfide-substituted amycolimiditide variants.
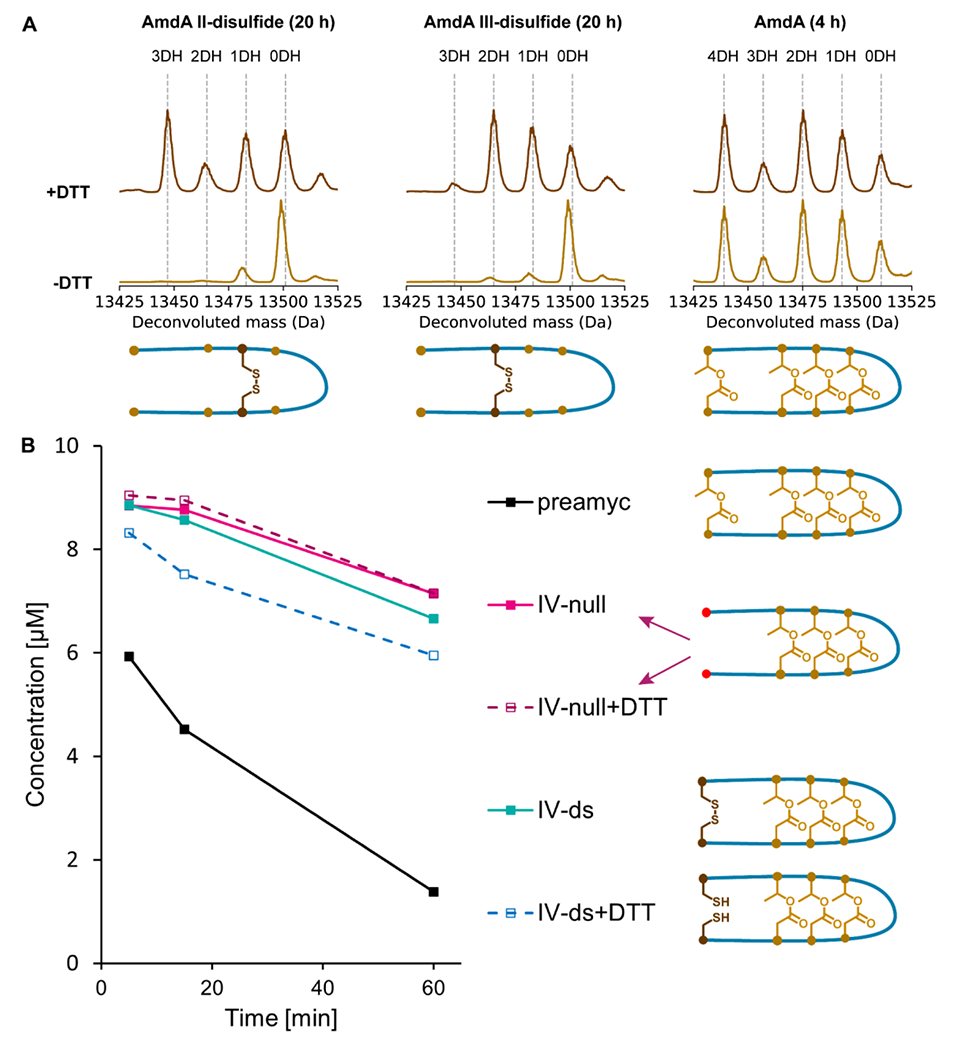
A: Esterification of disulfide-substituted AmdA variants in vitro by AmdB for 20 h at RT. The presence of a disulfide bond that constrains the peptide (-DTT spectra) inhibits esterification whereas reduced, unconstrained substrates are esterified efficiently. A reaction with wild-type AmdA (4 h at RT) is shown as a control. B: Time course of in vitro as partimidylation of pre-amycolimiditide variants with and without disulfide bonds by AmdM. All reactions were initiated with 10 μM peptide, but a mass spectrum was not acquired at the 0 min timepoint. The presence of disulfide bonds has minimal effect on the rate of methylation/aspartimidylation.
