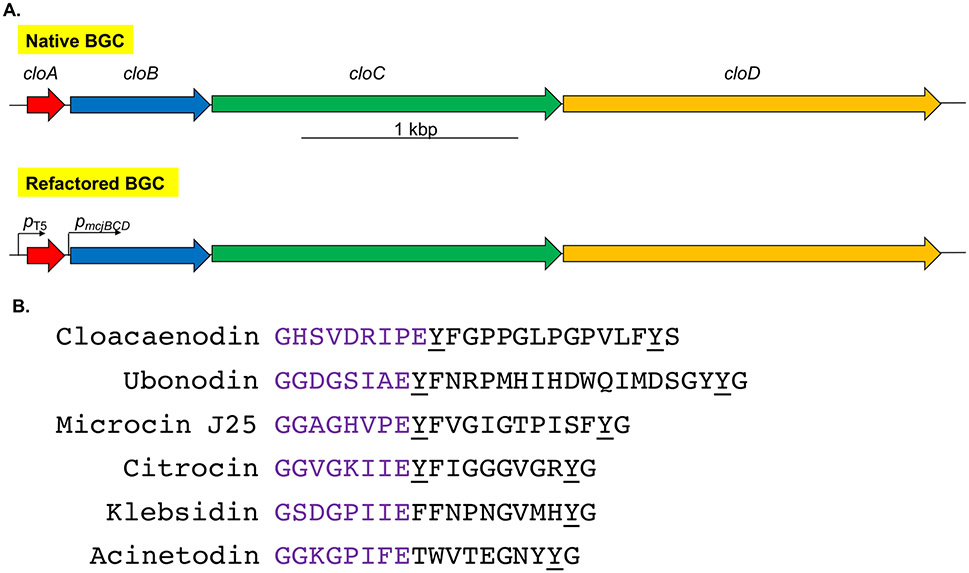
Figure 1.
Genome mining reveals a new lasso peptide from Enterobacter species. (A) The native BGC consists of a typical organization of lasso peptide genes in proteobacteria. It contains the precursor gene, cloA; the leader peptidase gene, cloB; the lasso peptide cyclase gene, cloC; and an ATP-binding cassette transporter gene for export of the mature lasso peptide, cloD. To facilitate heterologous expression, the BGC was codon-optimized and refactored into a pQE-80 vector with the cloA gene under the control of an IPTG-inducible T5 promoter, while the rest of the cluster is under the control of the constitutive pmcjBCD promoter. (B) The sequence of cloacaenodin and other known RNAP-inhibiting lasso peptides. Cloacaenodin contains a 9-membered ring (purple) and a C-terminal serine, which differs from the other lasso peptides which contain an 8-membered ring and a C-terminal glycine. Cloacaenodin contains the relatively well-conserved tyrosine residue directly after the ring, and the conserved penultimate tyrosine residue (underlined).