Figure 2. Achieving 7000+ targeted rare disease therapies – modeling future FDA orphan drug approval needs.
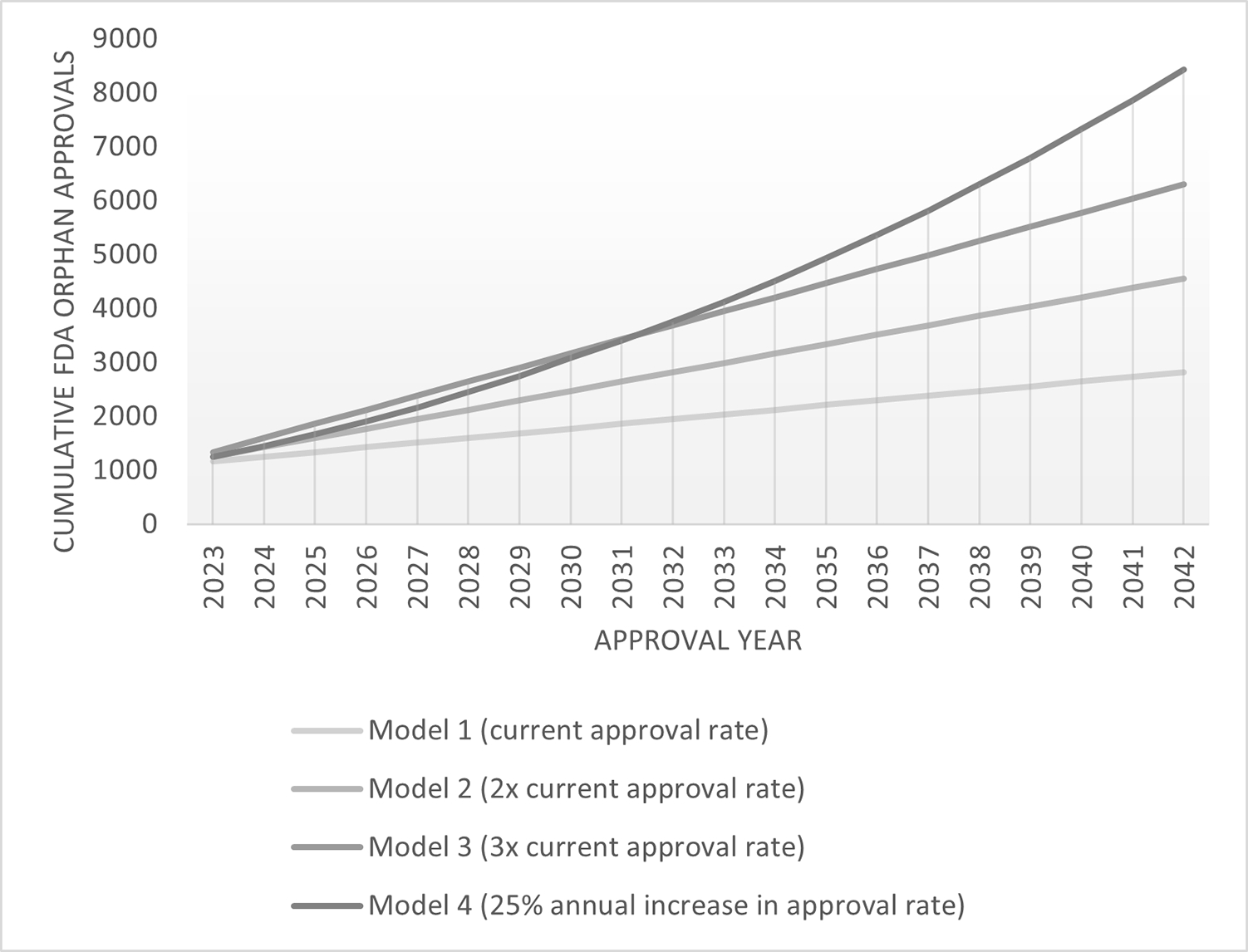
Projected cumulative FDA Orphan Drug approvals based on four annual approval rate models. Current approval rate is based on an average of 87 orphan drug approvals per year, 2017–2021. Data source, FDA (U.S. Department of Health and Human Services).
