Extended Data Figure 2. Validation of the AAV helper virus and recombinant rabies used in the retrograde connectomic pipeline in wild-type mice and non-neuronal Cre lines.
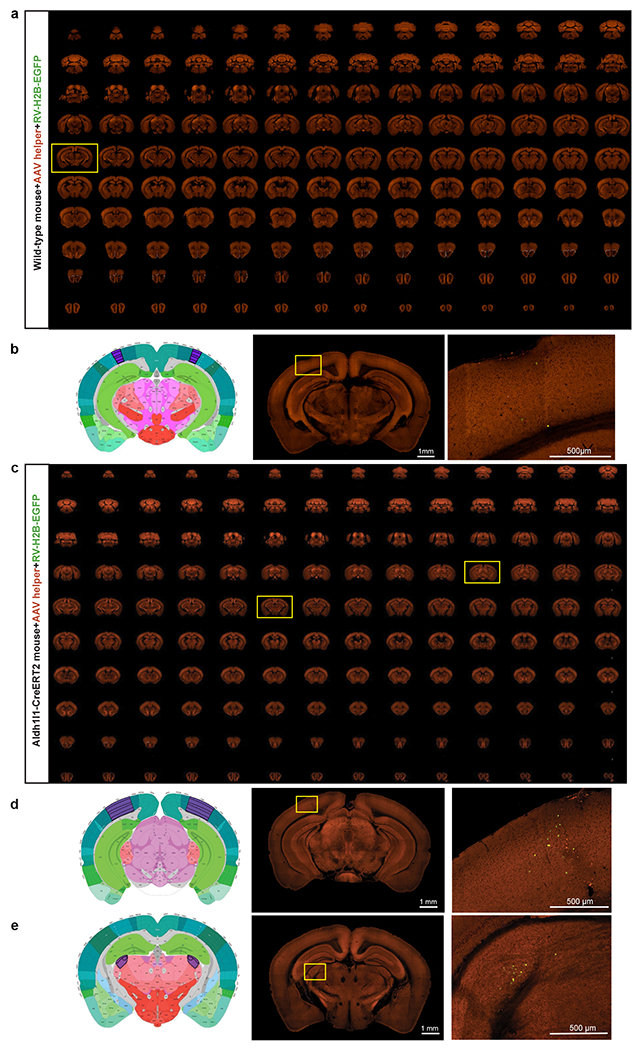
(a) Sequential two-photon images of a Cre-negative wild-type mouse brain injected with the AAV helper virus and EnvA-pseudotyped CVS N2cΔG rabies virus expressing H2B-EGFP. Similar results observed in 3 independent experiments. (b) Absence of RV-labeled neurons except a few H2B-EGFP-expressing cells in the injection site. Virus injection was targeted to VISp and validation was conducted in two wild-type mice. Left and middle panels: corresponding 2D atlas plate of Allen CCFv3 and the section image from the outlining box in a showing the injection site. Right panel: Image magnified from the box in the middle panel. Applying the monosynaptic rabies tracing to wild-type mice led to only a few H2B-EGFP-labeled cells in the injection site, but no starter cells in the injection site and no H2B-GFP-labeled cells outside the injection site. This shows that our system does not have the issue of spurious local rabies virus uptake due to low-level expression from the AAV helper in the absence of Cre, or local infection by small quantities of non-pseudotyped, RG-coated RVdG virus particles that may be present in the EnvA-pseudotyped rabies virus preparation. We then confirmed that the trans-synaptic transfer of the recombinant rabies relies on the expression of rabies G from the AAV helper. A G-minus version of the AAV helper virus, which conditionally expresses TVA66T and dTomato after Cre-mediated recombination, was injected into Cre+ mice, followed by the injection of rabies virus three weeks later. We observed H2B-EGFP-labeled cells only at the injection site and nowhere else in the brain. This finding confirms that the presynaptic labeling is specific for the Cre+ starter cells expressing the tricistronic cassette and infected with the RV-H2B-GFP rabies virus. (c) Sequential two-photon images of rabies labeling in an astrocyte-specific Cre mouse brain injected with hSyn promoter-driven AAV helper virus and recombinant rabies virus into VISp. Similar results observed in 4 independent experiments. (d-e) Left and middle two panels: corresponding 2D atlas plates of Allen CCFv3 and the section images from the boxes in c. Right panels: Representative images magnified from the boxes in the middle panels reveal sparse labeling around the injection site (d) and in LGd (e). We tested the monosynaptic rabies tracing system in three non-neuronal Cre lines, Olig2-Cre, Tek-Cre, and Aldh1l1-CreERT2, which express Cre in oligodendrocytes, vascular endothelium, and astrocytes, respectively. Among all experiments using the non-neuronal Cre lines, with either the hSyn-driven AAV helper virus used in the pipeline or a similarly constructed CMV-driven helper virus, sporadic long-distance H2B-EGFP-labeled cells were found only in 50% of the injected Aldh1l1-CreERT2 mice. Our results show that the occasionally infected non-neuronal cells do not support the spread of rabies virus to neurons in local or distant areas.
