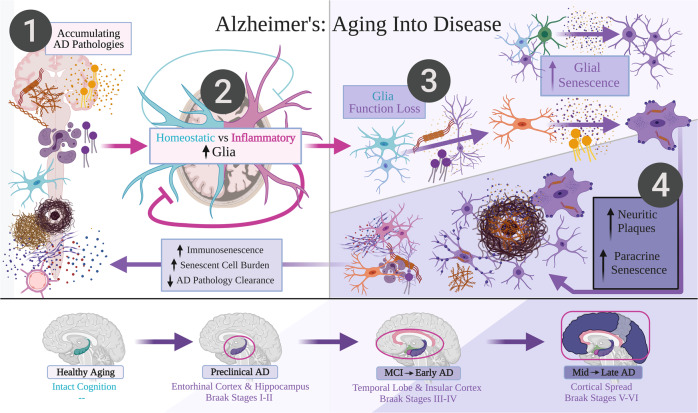
Fig. 2. Alzheimer’s: aging into disease.
A late-onset Alzheimer’s disease (LOAD) framework is proposed from healthy cognitive aging to late-stage Alzheimer’s disease (AD), presenting the following testable hypothesis: Glial senescence accumulation, particularly requiring increased senescent microglia burden, may correspond to clinical LOAD progression. Darker and deeper purple coloring denotes more severe senescent glia burden and Braak staging progression in LOAD. The hypothesis presents that: (i) Aging and AD pathologies from Fig. 1 (including amyloid-beta (Aβ), neurofibrillary pathology in hyper-phosphorylated tau (hp-tau), oxidative stress, and chronic inflammation) are predicted to progressively accrue throughout aging as by-products of central nervous system function and metabolism. (ii) Main AD pathology levels are likely enhanced by inflammatory glial states, and would be sufficiently cleared by glia performing homeostatic roles. However, homeostatic glial functions that putatively decline throughout aging would decrease proficiency in containing AD pathologies. (iii) In preclinical AD, increasing proportions of aged glia likely interact with AD pathologies to become senescent. This would include oligodendrocyte progenitor cells and astrocytes. Microglia performing homeostatic roles would also become senescent especially after engulfing neurons containing hp-tau. (iv) Senescent microglia then likely become incompetent in breaking down further phagocytosed hp-tau and Aβ aggregates, and instead secrete hp-tau to induce paracrine senescence in other microglia, especially those which are actively engulfing Aβ. Failure to suddenly degrade both hp-tau and Aβ likely induces secretion of Aβ and hp-tau aggregates that coalesce into neuritic amyloid plaques. These neuritic plaques correspond to Braak staging and clinical LOAD progression. Finally, this combination of senescent glia, reduced homeostatic glial support, and non-senescent, inflammatory glial states is predicted to drive neurodegeneration and cognitive decline in LOAD. While senescent glia burden is cleared out by immune cells, increasing immunosenescence over aging due to genetic and environmental circumstances likely slowly declines; thus, senescent glia burden is predicted to ultimately differentiate healthy cognition from mild cognitive impairment and LOAD. Figure created with BioRender.com.