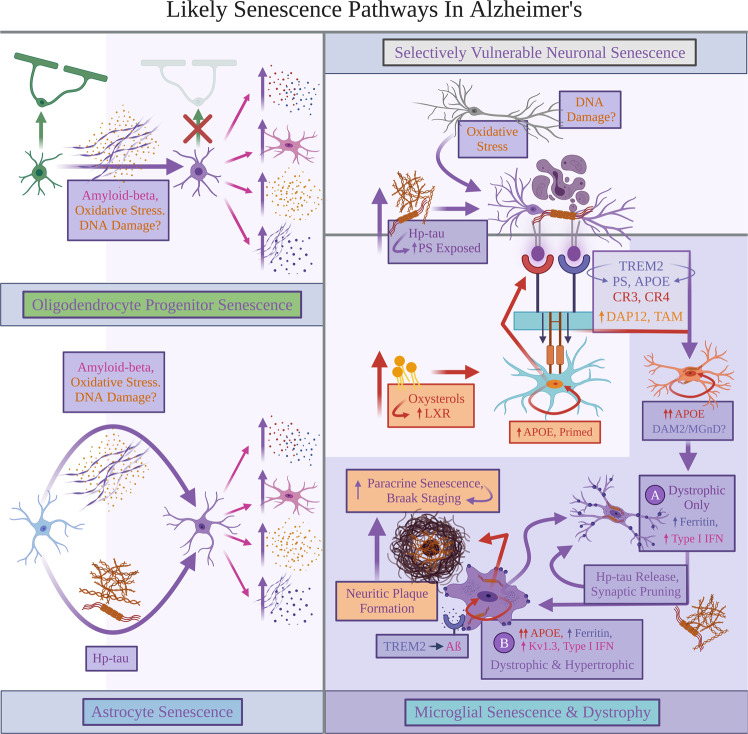
Fig. 3. Likely senescence pathways in Alzheimer’s.
Explanations for Alzheimer’s disease (AD) pathologies inducing senescence are proposed. Oligodendrocyte progenitor cells (OPCs) and astrocytes likely both react with amyloid-beta (Aβ) to become senescent. Oxidative stress and DNA damage may specifically lead to Aβ−induced senescence. Senescent OPCs and astrocytes are predicted to indirectly increase four pathologies: pro-inflammatory cytokines and reactive microglial states, oxidative stress, and Aβ accumulation. In neurons, oxidative stress, inflammation, and possibly DNA damage likely induce tau hyperphosphorylation; this process may also render the affected neurons senescent. Oxidative stress and hyperphosphorylated tau (hp-tau) aggregation likely prompt neurons to translocate and expose outer phosphatidylserine (PS). While aging predisposes sustained increases in local levels of inflammation, oxidative stress, and Aβ pathology burden, senescent OPCs and astrocytes are proposed to further worsen AD pathology. Senescent OPCs and astrocytes speculatively favor AD pathology buildup and environmental conditions that induce exacerbated microglial senescence in LOAD. Particularly, Aβ pathology contributes buildup to oxidative stress that oxidizes cholesterol to form oxysterols; these oxysterols may bind with liver-X-receptor expressed by homeostatic microglia states, resulting in upregulated apolipoprotein E (APOE) expression. Oxidative stress can also prime APOE-upregulated microglia to bind with neuronal PS using various receptors, enabling microglia to prematurely phagocytose and kill PS-exposed neurons containing hp-tau. This likely renders microglia senescent and unable to effectively phagocytose further AD pathologies, becoming proposed subtype or state “A” senescent microglia increasing type I interferon signaling, displaying dystrophic morphology, accumulating ferritin, and facilitating increased synaptic loss. They also proposedly secrete soluble hp-tau rendering paracrine senescence in nearby glia, thereby preceding neurofibrillary tangle accumulation in neurons. Local microglia attempting to actively phagocytose Aβ are predicted to be caught in this paracrine senescence feedback loop, becoming subtype or state “B” senescent microglia characterized by simultaneous dystrophy and a hypertrophic or “ameboid” appearance. We hypothesize that these hypertrophic, dystrophic, and senescent microglia secrete partially-digested aggregates that form neuritic plaques and further induce paracrine glial senescence. Finally, fewer homeostatic microglia proposedly remain available to degrade Aβ; Aβ would likely continuously accumulate and initiate increased pro-inflammatory responses, APOE, and KCNA3 upregulation in state “B” senescent microglia. Figure created with BioRender.com.