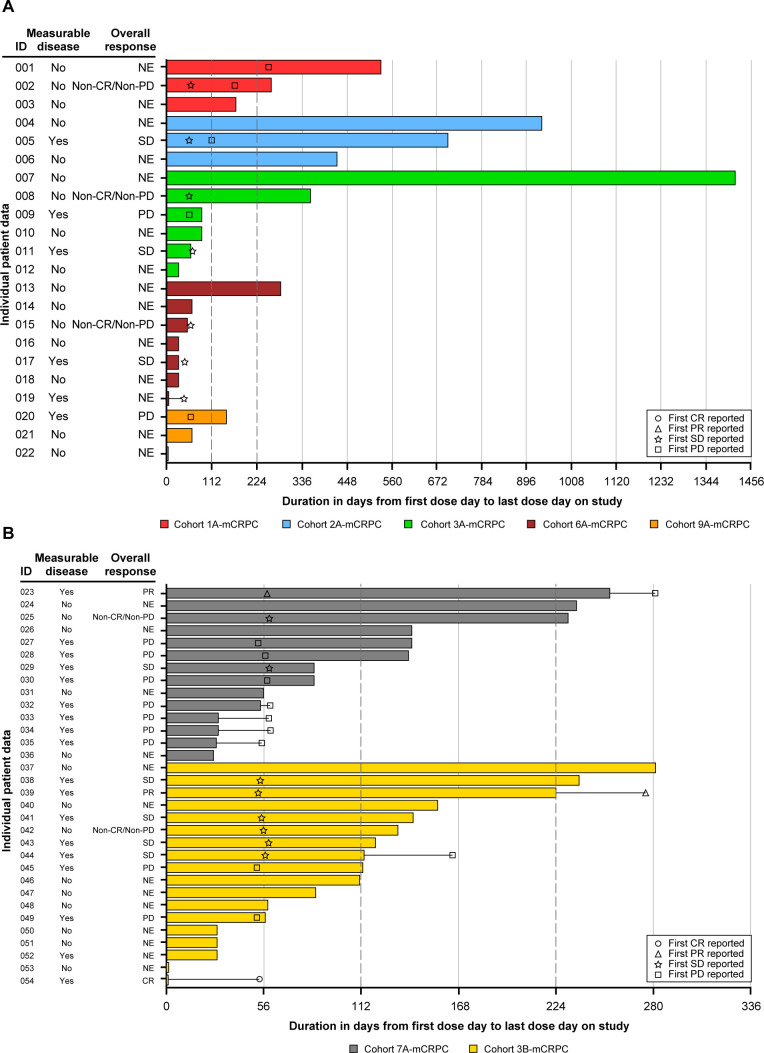
Figure 2.
Swimmer plot of duration of treatment and tumor response over time per RECIST V.1.1 in patients with mCRPC. (A) Patients with mCRPC excluding Cohorts 7A-mCRPC and 3B-mCRPC. (B) Patients with mCRPC in Cohorts 7A-mCRPC and 3B-mCRPC. mITT population. Treatment of each cohort see the footnote of table 2. Measurable disease at baseline was defined as showing new or progressive metastatic lymph node and/or local recurrence or visceral metastatic disease (with the exception of metastases to the liver) on CT or MRI scans. Duration of treatment was defined as (last dose date−first dose date+1). One patient from Cohort 3B-mCRPC achieved a confirmed CR (CR was 1.5-cm para-aortic lymph node, patient withdrew from study therapy after early Grade 3 diarrhea). CR, complete response; mCRPC, metastatic castration-resistant prostate cancer; mITT, modified intention-to-treat; NE, not evaluated; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.