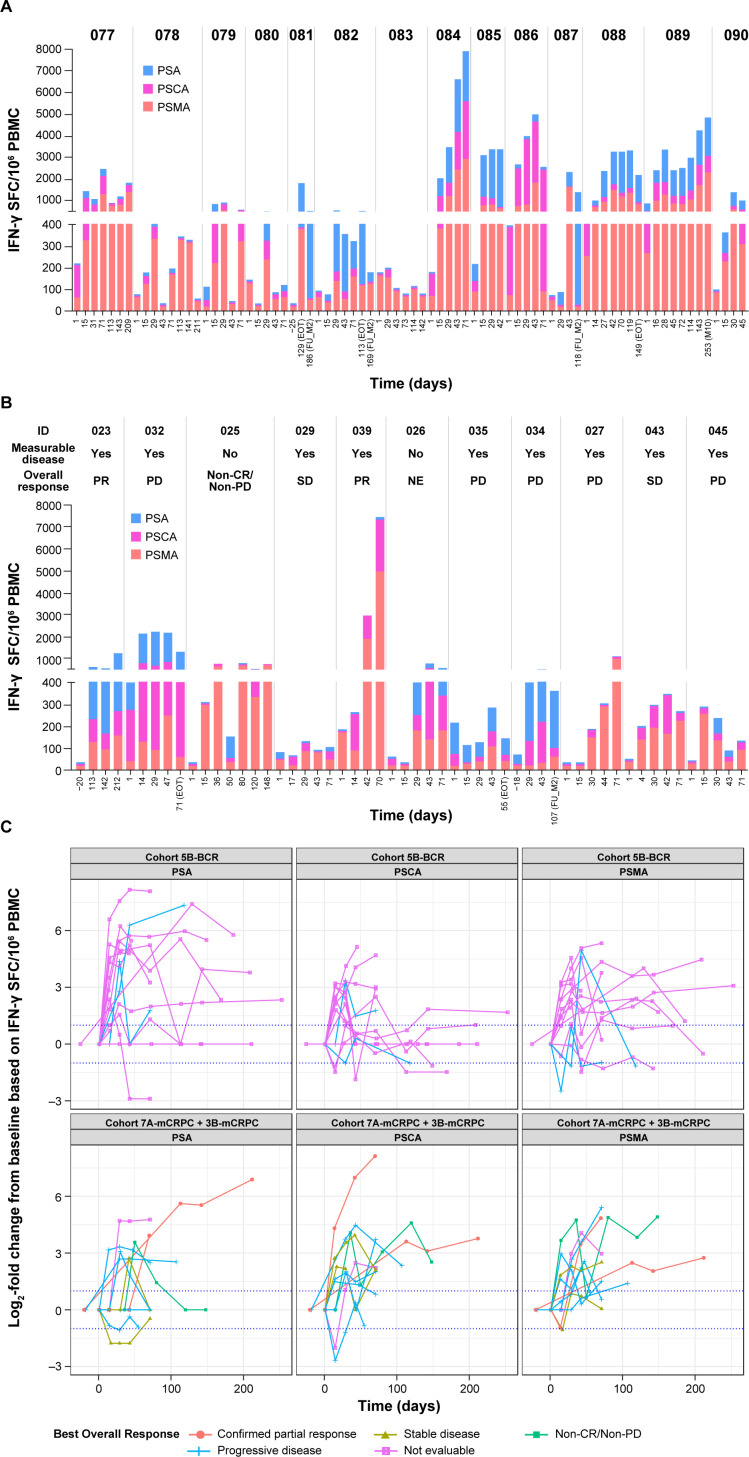
Figure 3.
T-cell immune response. (A) Patients with BCR in Cohort 5B. (B) Patients with mCRPC in Cohorts 7A and 3B. (C) Fold changes from baseline of each antigen. Patients with mCRPC in Cohorts 7A-mCRPC and 3B-mCRPC were treated at the RP2D, which was AdC68 6×1011 VP, the DNA booster vaccine, tremelimumab 80 mg, and sasanlimab 300 mg. For patients with BCR in Cohort 5B-BCR, the treatment was AdC68 6×1011 VP, the DNA booster vaccine, tremelimumab 80 mg, and sasanlimab 130 mg.BCR, biochemical recurrence; CR, complete response; EOT, end of treatment; IFN, interferon; mCRPC, metastatic castration-resistant prostate cancer; NE, not evaluated; PBMC, peripheral blood mononuclear cell; PD, progressive disease; PR, partial response; PSA, prostate-specific antigen; PSCA, prostate stem cell antigen; PSMA, prostate-specific membrane antigen; RP2D, recommended phase 2 dose; SD, stable disease; SFC, spot-forming cells; VP, viral particle.