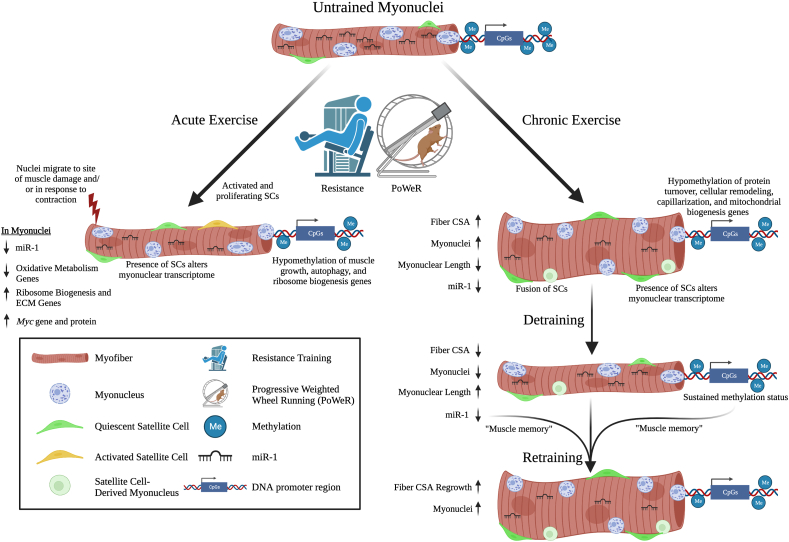
Fig. 1.
With acute exercise, myonuclei can migrate along the myofiber to a site of damage and aid in localized mRNA delivery to facilitate muscle sarcomere repair. Myonuclear movement is likely of particular relevance with unaccustomed exercise featuring an eccentric (damaging) component, but some evidence also suggests that myonuclei can move as a consequence of contraction. Satellite cells activate and may proliferate in response to several acute exercise-induced signals. The presence of satellite cells can also influence the myonuclear transcriptome independent from fusion, as revealed by satellite cell loss-of-function studies. Epigenetic modifications that occur in myonuclear DNA after exercise include hypomethylation in promoter regions of genes involved in muscle growth, autophagy, and ribosomal biogenesis, along with lower expression of miR-1. Epigenetic modifications in muscle fibers may result in transiently reduced expression of oxidative metabolism genes, as well as increased expression of extracellular matrix and ribosomal biogenesis genes. With chronic exercise training, myofiber size and myonuclear density increase due to fusion of satellite cells, and a decrease in myonuclear length. The myonuclear transcriptome may be altered by the presence of satellite cells and epigenetic modifications to myonuclear DNA such as hypomethylation of genes involved in protein turnover, cellular remodeling, capillarization, and mitochondrial biogenesis, as well as chronically reduced expression of miR-1. Epigenetic modifications are sustained with detraining and may function as a “muscle memory” to potentiate a rapid re-acquisition of training adaptations upon retraining. Figure was generated using BioRender.